Background
Aneurysmal subarachnoid hemorrhage (SAH) is one of the most frequent neurological emergencies to require admission to an intensive care unit (ICU). Reference Suarez, Martin and Bauza1 SAH severity is commonly expressed using the World Federation of Neurological Surgeons (WFNS) grading system, ranging from a minimum score of 1 (no neurological deficit) to a maximum of 5 [comatose, with Glasgow Coma Scale (GCS) score of 3 to 6 (motor response to noxious stimulation no better than withdrawal)]. 2 The Hunt–Hess (HH) grading system is similar but has inferior interobserver agreement. Reference Degen, Dorhout Mees, Albra and Rinkel3 Grade 5 SAH is a life-threatening condition with high risk of death and long-term disability, but cohort studies have reported that a proportion of patients may ultimately achieve favorable outcomes. Reference van den Berg, Foumani and Schroder4-Reference Haug, Sorteberg and Finset13
Accurate and timely assessment of neurological prognosis, coupled with transparent conversations with patients’ families, is a challenging, but necessary, aspect of providing critical care to patients with grade 5 SAH. If there is no realistic hope of favorable recovery, surgical intervention to treat the source of SAH will have limited benefit, and some patients may instead prefer (as expressed through an advanced directive or surrogate decision-maker) to transition to withdrawal of life-sustaining measures (WLSM). Conversely, premature and unjustified pessimism by healthcare providers has potential to produce “self-fulfilling prophecy,” whereby patients invariably die following WLSM, even if they were not necessarily destined for a poor outcome. Reference Kowalski, Chang and Carhuapoma14-Reference Becker, Baxter and Cohen16
While there are clinical and radiographic factors that have been identified as being predictive of poor outcome following SAH, these are not sufficiently accurate to be definitive and are not specifically for patients with grade 5 SAH, which is the subgroup where WLSM is most often considered. Reference Rosengart, Schultheiss, Tolentino and Macdonald17,Reference Jaja, Cusimano and Etminan18 Published consensus guidelines for management of patients with SAH do not provide clear recommendations regarding determination of neurological prognosis. Reference Connolly, Rabinstein and Carhuapoma19-Reference Steiner, Juvela and Unterberg21
We performed a cohort study involving consecutive patients with WFNS grade 5 SAH over a period of about 10 years for two purposes: (1) to assess factors associated with poor prognosis, particularly those with high positive predictive value (PPV, ≥ 90–95%) (or, stated differently, with < 5–10% “false positive” rate, where poor outcome was incorrectly predicted); and (2) to assess factors associated with a decision for WLSM, which could theoretically contribute to self-fulfilling prophecies and would confound the utility of a particular prognostic variable in predicting poor outcome.
Methods
This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary.
Patients with WFNS grade 5 SAH were identified using a prospectively maintained neurocritical care database at the Foothills Medical Center, a neurosurgical facility serving a population of about 2.5 million inhabitants of southern Alberta and southeastern British Columbia. The database records aneurysm size, method of treatment (endovascular or surgical), radiographic characteristics, and details regarding identification and treatment of delayed cerebral ischemia (DCI). Intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH) volumes are estimated using the ABC/2 method and IVH score, respectively. Reference Kothari, Brott and Broderick22-Reference Kramer, Mikolaenko and Deis24 Cisternal hematoma volume is quantified using the SAH sum score. Reference Hijdra, Brouwers and Vermeulen25 Extent of ventriculomegaly is assessed using the bicaudate index. Reference van Gijn, Hijdra and Wijdicks26 All of these variables are prospectively adjudicated by neurocritical care specialists.
Critical care of patients with SAH is directed by local consensus guidelines, which are in turn based on international guidelines. Reference Connolly, Rabinstein and Carhuapoma19,Reference Diringer, Bleck and Hemphill20 Admission to the ICU and insertion of an external ventricular drain (EVD) is standard for patients who do not meet criteria for neurological determination of death (NDD) or present with loss of multiple brainstem reflexes. All patients are treated with nimodipine. Endovascular coil embolization or surgical clip ligation is generally performed within 24 hours for patients who have a motor response better than extensor posturing. WLSM decisions are not protocolized but are based on individualized, collaborative conversations of critical care and neurosurgery teams with patients’ families.
Neurological outcomes are recorded utilizing questionnaires sent to patients and their families, and through follow-up visits with the most responsible neurosurgeon and physiatrist, which are documented in the provincial electronic medical record. One-year outcomes for this study were prospectively adjudicated, initially by a trained research coordinator (SR), with independent validation by a neurocritical care specialist (AK), and discrepancies resolved by the involvement of another neurocritical care specialist (JK). A modified Rankin Score (mRS) of 0–3 was considered to indicate a favorable recovery. Reference Patel, Rao and Heilman_Espinoza27
The above information was linked with eCritical Alberta, which consists of a clinical information system (Meta Vision, iMDsoft, Tel Aviv) and data warehouse (TRACER) used for all critically ill patients in Alberta. TRACER records daily GCS scores determined by the attending physician and all GCS scores and pupillary light reflexes (PLRs) documented by bedside critical care nurses every 1–4 hours. For this study, we chose only GCS scores assessed in the absence of sedation. If the unconfounded GCS motor score varied within 3 hours before or after the time period of interest, we used the highest score.
Characteristics of patients with favorable versus unfavorable outcomes were compared using chi-square analysis and Kruskal–Wallis tests. Multivariable models were created using backward elimination of variables with p-values > 0.10.
A “true positive” (TP) outcome prediction was defined as occurring when a particular clinical finding was present and correctly predicted mRS of 4–6. A “false positive” (FP) occurred when a finding predicted poor outcome but the patient actually had a favorable recovery. A “true negative” (TN) was defined as occurring when a clinical finding correctly predicted a favorable recovery. A “false negative” (FN) occurred when a clinical finding predicted a favorable recovery, but the patient actually had an unfavorable outcome. Sensitivity [TP/(TP+FN)], specificity [TN/(TN/FP)], PPV [TP/(TP+FP], and negative predictive value (NPV) [TN/TN+FN], with corresponding 95% confidence intervals, were calculated for each variable of interest.
Because unfavorable clinical findings are used to inform crucial decisions regarding WLSM, they should have high PPV and specificity (low false positive rate). A 95% PPV threshold has been considered relevant in neuro-prognostication guidelines among cardiac arrest survivors and was the primary outcome of our study. Reference Sandroni, Cariou and Cavallaro28 As a secondary analysis, we also included variables with ≥ 90% PPV. All statistical analyses were performed using SAS (Version 9.4, Cary, NC). p-Values < 0.05 were considered statistically significant.
To assess the concept of self-fulfilling prophecy in neuroprognostication, we reviewed the medical records of all patients in whom life-sustaining measures had been withdrawn. They were divided into two categories: (1) patients who were moribund with severe cerebral edema, manifesting with either clinical and radiographic evidence of transtentorial or tonsillar herniation, or refractory intracranial hypertension; and (2) patients in whom there was no evidence of the above, but WLSM was directed by “shared decision making” with patients’ families, based on the belief that the prognosis was sufficiently poor that ongoing life-sustaining measures would not be consistent with patients’ preferences. In the former patients, there is relatively less controversy that the prognosis is poor and there is less potential for the concept of self-fulfilling prophecy. Reference Fung, Inglin and Murek29 Division into these two categories was performed independently by two critical care physicians, with disagreements resolved by consensus, and initial interrater agreement assessed using Cohen’s kappa statistic. Scores of 0.60–0.79 and 0.80–1.00 indicate moderate and strong agreement, respectively. Reference McHugh30 Characteristics of patients undergoing WLSM were compared to those who continued to receive life-sustaining measures to assess factors that may have influenced the decision to change the goals of care.
To compare our findings with previous publications, we searched English language literature in Medline from 2000 to September, 2020 to identify other studies assessing prognosis specifically among patients with WFNS or HH grade 5 SAH. The MeSH term “subarachnoid hemorrhage” was combined with key words “WFNS,” “World Federation of Neurological Surgeons,” “Hunt Hess,” “outcome,” “survival,” “mortality,” “prognosis,” and “recovery.”
Results
Predictors of Outcome in WFNS Grade 5 SAH (Tables 1 and 2)
Table 1: Characteristics of patients with World Federation of Neurosurgeons grade 5 subarachnoid hemorrhage, dichotomized based on 1-year neurological outcome
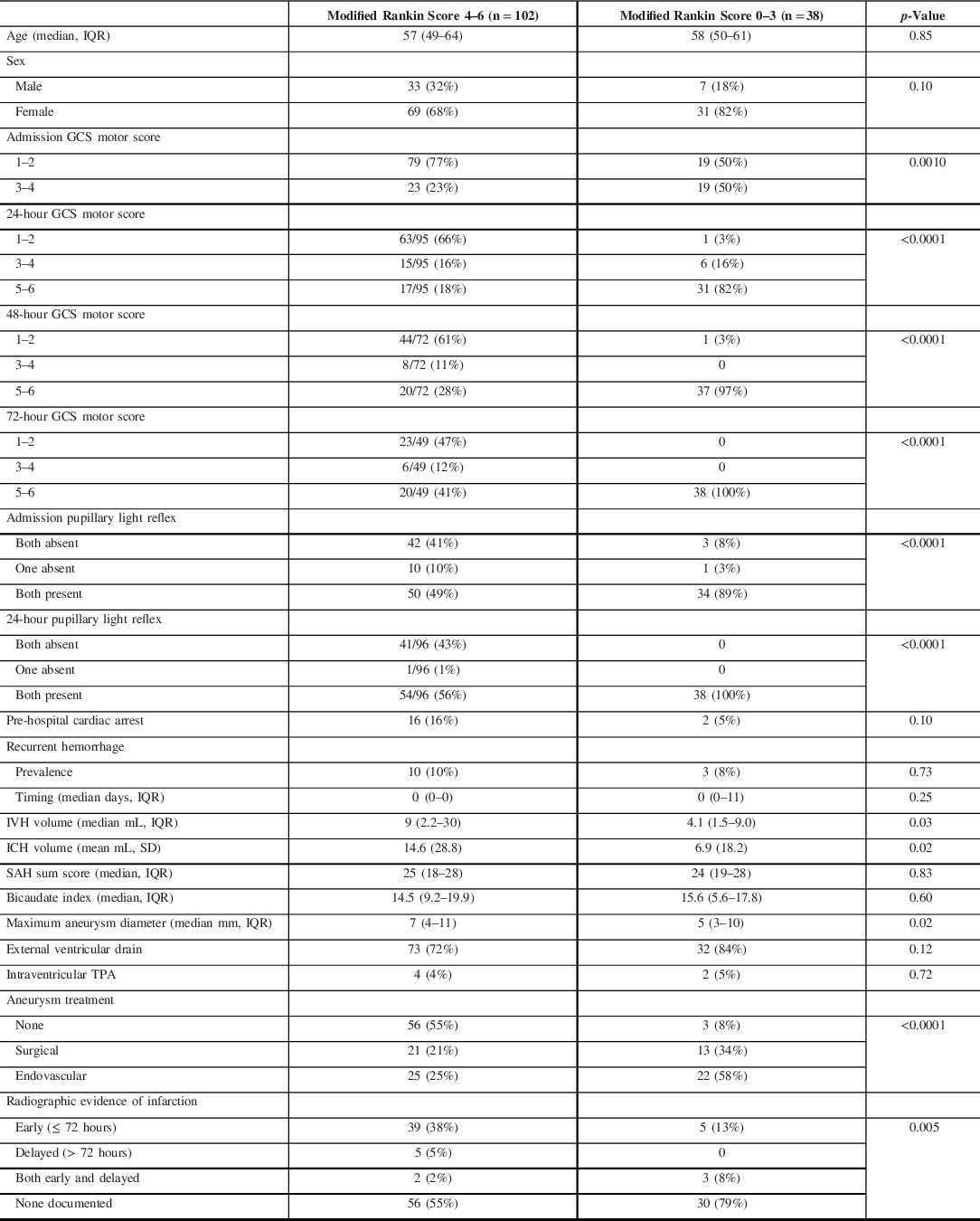
GCS = Glasgow Coma Scale; ICH = intracerebral hemorrhage; IQR = interquartile range; IVH = intraventricular hemorrhage; SAH = subarachnoid hemorrhage; SD = standard deviation; TPA = tissue plasminogen activator
Table 2: Multivariable analysis of factors associated with poor outcome following World Federation of Neurological Surgeons grade 5 subarachnoid hemorrhage
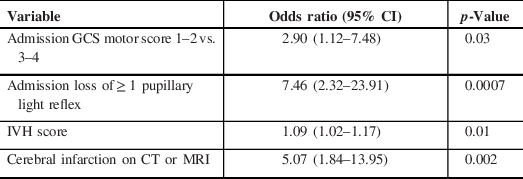
CI = confidence intervals; CT = computer tomography; GCS = Glasgow Coma Scale; IVH = intraventricular hemorrhage; MRI = magnetic resonance imaging
Between November, 2008 and June, 2019, there were 140 patients with WFNS grade 5 SAH admitted to the ICU, all of whom were mechanically ventilated. ICU and hospital mortality were 59% and 63%, respectively. A favorable outcome (mRS ≤ 3) occurred in 38 (27%).
The following factors were significantly predictive of poor outcome: lower GCS motor score at admission (p = 0.001) and on each of the first 3 days in ICU (p < 0.0001); loss of at least one PLR at admission and at 24 hours post-admission (p < 0.0001); volume of IVH (p = 0.03); presence and volume of ICH (p = 0.02); larger maximal aneurysm diameter (p = 0.02); and radiographic documentation of cerebral infarction (p = 0.005) (Table 1).
In multivariable analysis, using time of admission for repeated variables (motor score and pupillary light reactivity), independent predictors of poor outcome included lower GCS motor score (odds ratio [OR] 2.90, p = 0.03 for score 1–2 vs. 3–4), loss of at least one PLR (OR 7.46, p = 0.0007), IVH score/volume (OR per point 1.09, p = 0.01), and radiographic identification of cerebral infarction (OR 5.07, p = 0.002) (Table 2).
Variables with High PPV for Poor Outcome (Table 3)
Table 3: Clinical findings associated with ≥ 90% positive predictive value for poor outcome in patients with World Federation of Neurological Surgeons grade 5 subarachnoid hemorrhage

CI = confidence intervals; GCS = Glasgow Coma Scale; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; NPV = negative predictive value; PPV = positive predictive value
Although an admission motor score of 1–2 (no motor response or abnormal extension with noxious stimulation) was strongly associated with poor outcomes, it was not uncommon for such patients to eventually have favorable recovery (19/98, 19%). In all cases where this occurred, patients had improved to a motor score of at least 5 (localization to noxious stimulation) or 6 (following one-step commands) by 72 hours (Table 1). Similarly, some patients with loss of at least one PLR at admission ultimately had favorable outcomes (4/56, 7%), but pupillary reactivity had invariably returned in these cases by 24 hours (Table 1).
Sensitivity, specificity, PPV, and NPV values in the prediction of poor outcome are shown in Table 3. Findings with ≥ 95% PPV for poor outcome (< 5% or patients with the finding had a favorable recovery) included GCS motor score ≤ 4 at 72 hours, absence of at least one PLR at 24 hours, and IVH score ≥ 20 (corresponding to IVH Volume ≥ 54.6 mL). ICH of volume ≥ 53 ml and aneurysm of diameter ≥ 1.6 cm both had high specificity of > 95%, but slightly lower PPV (92% and 89%, respectively).
Mechanism of Death and Factors Associated with WLSM (Figure 1; Table 4)

Figure 1: Outcomes of patients with World Federation of Neurological Surgeons grade 5 subarachnoid hemorrhage. mRS = modified Rankin Score (0–3 considered a favorable recovery); SAH = subarachnoid hemorrhage; WFNS = World Federation of Neurological Surgeons; WLSM = withdrawal of life-sustaining measures.
Table 4: Characteristics of patients with World Federation of Neurological Surgeons grade 5 subarachnoid hemorrhage divided based on whether they underwent withdrawal of life-sustaining measures

GCS = Glasgow Coma Scale; ICH = intracerebral hemorrhage; IQR = interquartile range; IVH = intraventricular hemorrhage; NDD = neurological determination of death; SAH = subarachnoid hemorrhage; SD = standard deviation; WLSM = withdrawal of life-sustaining measures
* Statistically significant difference also between categories 3 and 4
Thirty-three (24%) patients met criteria for NDD after a median of 1 day (interquartile range (IQR) 0–1; range 0–11 days) (Figure 1). Another 28 (20%) underwent WLSM after a median of 2 days (IQR 1–3; range 0–6 days) because they had evidence of severe cerebral edema despite aggressive medical therapy, together with evidence of herniation (transtentorial or tonsillar) or refractory intracranial hypertension.
Of the remaining 83 patients, another 25 (18%) eventually underwent WLSM based on the perception that the anticipated outcome was not consistent with their treatment preferences. This occurred after a median of 5 days (IQR 3–9; range 1–18 days). All of these patients died. Of 54 patients in whom life-sustaining measures were continued, 38 (70%) ultimately had a favorable outcome. Reviewers agreed on the reason for WLSM in 47 of 53 patients (89%), yielding a kappa statistic of 0.77 (95% CI 0.60–0.94), which is in the “moderate” range for interobserver agreement.
Compared with patients who underwent WLSM because of a perceived poor prognosis (column 3, Table 4), those who did not (column 4, Table 4) had significantly higher GCS motor scores at admission (p = 0.006) and on each of first 3 days post-SAH (p < 0.0001 for each day) and were less likely to have developed radiographic evidence of infarction (p = 0.02). There were no significant differences in age (p = 0.50), sex (p = 0.78), pupillary reactivity (p = 0.51, 0.07, and 0.13 at admission, 24 hours, and 48 hours, respectively), pre-hospital cardiac arrest (p = 0.40), rebleeding (p = 0.18), IVH score (p = 0.07), ICH volume (p = 0.30), cisternal SAH sum score (p = 0.79), bicaudate index (p = 0.29), or aneurysm diameter (p = 0.82). The vast majority of patient who did not meet criteria for NDD at admission had an initial trial of therapy with either placement of a ventricular drain, aneurysm surgery, or both (89%, 96%, and 100% for columns 2, 3, and 4, respectively). With exclusion of patients who underwent WLSM because of perceived poor prognosis (column 3 of Table 4), the variables with ≥ 90% PPV for poor outcome did not change.
Characteristics of WFNS Grade 5 Patients with Favorable Outcomes (Table 1)
Patients with favorable 1-year outcomes had a median age of 58 (50–61) years and were more often female (82%). Two patients sustained cardiac arrests in the pre-hospital setting, with both achieving rapid return of spontaneous circulation. IVH was present in 89% (median 3.3, 1.5–9.0 ml) and ICH was present in 16% (median 46, 33–52 ml). Ventriculomegaly was judged to present in 63%, although the bicaudate index exceeded the age-specific upper limit of normal in only 18%.
An EVD was inserted in 84% at a median of 129 (101–204) minutes after the diagnosis of SAH and 13% underwent emergency craniotomy to remove ICH. The most common aneurysm locations were anterior communicating (32%) and middle cerebral (29%) arteries. Treatment consisted of endovascular coil embolization in 58% and surgical clipping in 34%, which were provided a median of 13 (5–19) hours after diagnosis.
Twenty-six (68%) patients developed radiographic evidence of vasospasm, of which 12 (32%) developed DCI at a median of 6 (4.5–7) days after SAH. Twelve patients (32%) were treated with induced hypertension using norepinephrine, 6 (16%) with intravenous milrinone, 5 (13%) with intra-arterial vasodilators, and 3 (8%) with balloon angioplasty. Three patients (8%) developed delayed infarction.
Patients were mechanically ventilated for a median of 7 (2–13) days. ICU and hospital (including in-patient neurorehabilitation) length of stay were 12 (5–17) and 76 (26–118) days, respectively.
Patients with Major Improvement Following EVD Insertion
There were 22 patients who had an EVD inserted and were subsequently following simple one-step commands within the next 24 hours, of which 18 (82%) had a favorable 1-year outcome. Data were re-analyzed with exclusion of these 22 patients.
Variables predictive of poor outcome in univariate analysis (Table 1) remained similar, except that the differences in median IVH and ICH volumes were no longer statistically significant. Similarly, in multivariable analysis (Table 2), IVH volume was no longer significantly predictive of poor outcome (OR per point 1.06, p = 0.14), while a lower GCS motor score (OR 5.93, p = 0.003 for score 1–2 vs. 3–4), loss of at least one PLR (OR 8.12, p = 0.009), and radiographic evidence of infarction (OR 3.85, p = 0.03) continued to be.
Variables with greater than 90–95% PPV for poor outcome remained the same, although the sensitivity of a 72-hour GCS motor score ≤ 4 was substantially higher among EVD nonresponders (sensitivity 81%, 71–88%). In addition, no patient with an aneurysm diameter ≥ 1.4 cm had a favorable outcome (sensitivity 15%, 9–24%; PPV 100%, 75–100%).
Discussion
WFNS grade 5 SAH is a devastating condition with a high mortality rate and significant disability among survivors. Nevertheless, in this relatively large cohort of consecutive patients over more than a decade, we found that 38% of patients were discharged from hospital alive and 27% had favorable long-term outcomes. Because of the potential for favorable recovery, it is essential to provide appropriate initial resuscitation, including mechanical ventilation, hemodynamic support, and treatment of hydrocephalus and cerebral edema.
To aid critical care and neurosurgery teams, as well as patients’ families, in decision-making, an understanding of factors that are predictive of poor outcome with few “false positives” (wrongly suggesting a poor prognosis) would be helpful. In the setting of hypoxic-ischemic brain injury following a cardiac arrest, clinical and neurophysiological findings (e.g., absence of pupillary light or corneal reflexes at ≥ 72 hours and absence of N20 somatosensory evoked potential responses at 24–72 hours) are well known to be highly predictive of poor outcome. Reference Sandroni, Cariou and Cavallaro28,Reference Callaway, Donnino and Fink31 Surgical treatment of aneurysms and life-sustaining measures in an ICU are invasive and expensive and would not be appropriate for patients who definitively have a poor prognosis. While prognostic scales do exist for patients with SAH, these are not specifically for those with WFNS or HH grade 5. Reference van Donkelaar, Bakker and Birks32-Reference Lee, Ouyang and John35 Consideration of WLSM is much less common with lower-grade SAH, such that there is not the same need for earlier assessment of prognosis.
We identified clinical and radiographic findings with ≥ 95% PPV for poor outcome. These included an unconfounded GCS motor response ≤ 4 (withdrawal to noxious stimulation) at 72 hours, absence of at least one PLR at 24 hours, and IVH score ≥ 20 (estimated volume ≥ 54.6 mL). The lower limit of the 95% confidence interval for these findings ranged from 79% to 90%. Although it did not reach our primary outcome threshold of ≥ 95% PPV, ICH volume of ≥ 53 mL also had high PPV (92%) and specificity (97%). GCS motor response ≤ 4 at 72 hours had a higher sensitivity (59%) compared with the other findings.
In some patients with WFNS grade 5 SAH, a major cause of coma is the development of hydrocephalus rather than the severity of early brain injury. Patients who improve following cerebrospinal fluid drainage may have a prognosis similar to patients with lower-grade SAH. Reference Alotaibi, Elkarim and Samuel36 In our cohort, a majority of patients who were able to follow commands within 24 hours of EVD insertion had favorable recovery. However, with exclusion of these patients from the analysis, our predictors with high PPV for poor outcome were the same, with the addition of aneurysm diameter of ≥ 1.4 cm.
An important potential limitation is that some of these variables may have been factors contributing to decisions to proceed with WLSM. In particular, a 72-hour GCS motor score ≤ 4 was a statistically significant predictor of WLSM and was observed in 70% of patients in whom the WLSM decision was based largely on conversations with patients’ surrogate decision-maker(s) regarding goals of care (category 3 in Table 4). Although WLSM occurred significantly later compared to patients with severe cerebral edema (category 2 in Table 4) (p < 0.0001) and as late as 2–3 weeks post-SAH in some cases, it remains possible that outcomes may sometimes have been favorable had these patients continued with life-sustaining measures. Loss of pupillary reactivity and large IVH and ICH volume were not statistically significant factors associated with WLSM decisions.
For patients with refractory cerebral edema despite aggressive treatments (category 2 in Table 4), there is relatively less potential for self-fulfilling prophecy. Among these patients, 89% underwent placement of either a ventricular drain or emergency craniotomy for evacuation of ICH. A potential limitation is that it was rare for patients in our cohort to undergo decompressive craniectomy, which is a potentially life-saving treatment for severe cerebral edema. Although not demonstrated to be beneficial in any clinical trials of SAH patients, and not recommended in guidelines, patients undergoing decompressive craniectomy have been reported to sometimes achieve favorable outcomes. Reference Ransom, Mocco and Komotar37 Although only about half of the patients who died following WLSM had undergone preceding aneurysm treatment, this is unlikely to have influenced our results since rebleeding was uncommon.
For patients with very large volume of IVH, it remains unclear whether the use of intrathecal thrombolytics to achieve rapid clearance of blood might improve prognosis. Reference Kramer and Fletcher38 Only 4% of patients in our study received intraventricular tissue plasminogen activator. Similarly, for some patients with concomitant SAH and large ICH volume, it is conceivable that emergent evacuation together with aneurysm clipping may modify outcomes. Reference Wan, Jaja and Schweizer39 Among 13 patients with ≥ 53 ml hematoma volume, 8 (62%) underwent emergency surgery, of which only 1 had a favorable outcome.
Ischemic strokes were detected by either computer tomography (CT) or magnetic resonance imaging (MRI) in over 60% of cases where WLSM occurred. Most infarcts occurred within 72 hours, prior to any documented vasospasm or DCI, demonstrating the importance of “early brain injury” in contributing to poor outcomes among patients with high-grade SAH. Reference Sehba, Hou, Pluta and Zhang40 A study of grade 4 and 5 SAH patients who underwent MRI scanning found that about two-thirds had evidence of early ischemia. Reference Frontera, Ahmed and Zach41 Apart from resuscitation aimed at ensuring adequate gas exchange and cerebral perfusion pressure, as well as treatment of cerebral edema, there are no therapies known to prevent early brain injury. Detection of infarction was not a primary aim of our study, such that this was not prospectively and systematically sought and may therefore have been underestimated. Furthermore, infarction was recorded as a dichotomous outcome, but infarction volume is likely a more important factor.
Although not statistically significant in our study, older age is reported in the literature to be associated with decisions to proceed with WLSM following grade 5 SAH. Reference Kowalski, Chang and Carhuapoma14 Also, a study utilizing the US National Inpatient Sample suggested that there may be factors other than severity of SAH, including demographic characteristics, that contribute to WLSM decisions. Reference Qureshi, Adil and Suri42
Similar to our experience, other cohort studies have reported that essentially all grade 5 SAH patients who undergo WLSM die, and that WLSM precedes the majority of deaths. Reference Hoogmoed, de Oliveira Manuel and Coert43,Reference Lantigua, Ortega-Guitterez and Schmidt44 These findings highlight the importance of having validated and accurate prognostic tools to inform conversations regarding end-of-life care. A propensity analysis from one center provides some reassurance, having found that patients who received continuation of life-sustaining measures despite having characteristics similar to those undergoing WLSM almost invariably had poor neurological outcomes. Reference Weimer, Nowacki and Frontera45
Our literature review found few previous studies that have identified variables with high PPV for poor outcome among patients with grade 5 SAH (Table 5). Consistent with our findings, Van den Berg and colleagues reported that 72-hour GCS motor score ≤ 3 had 100% PPV for poor outcome. Of note, GCS motor scores of 3 and 4 are often difficult to distinguish, with considerable potential for interobserver variability, which is why the FOUR score, an alternative coma scale to the GCS, classifies them together. Reference Wijdicks, Bamlet and Marmattom46 Other studies have variably reported that high mean transit time on CT perfusion scans, evidence of early ischemic changes on CT angiography, absent corneal reflexes, and vasospasm-induced infarction are also highly predictive of poor outcome. Reference Konczalla, Seifert and Beck5,Reference Ariyada, Ohida and Shibahashi9,Reference Sasahara, Suzuki and Takahashi12,Reference Sorimachi, Osada and Aoki47 In keeping with our results, several studies indicate that even ominous admission characteristics, such as absent pupillary light reactivity or pre-hospital cardiac arrest, do not have sufficient PPV to predict hopeless outcome.
Table 5: Cohort studies assessing prognosis specifically in grade 5 subarachnoid hemorrhage patients
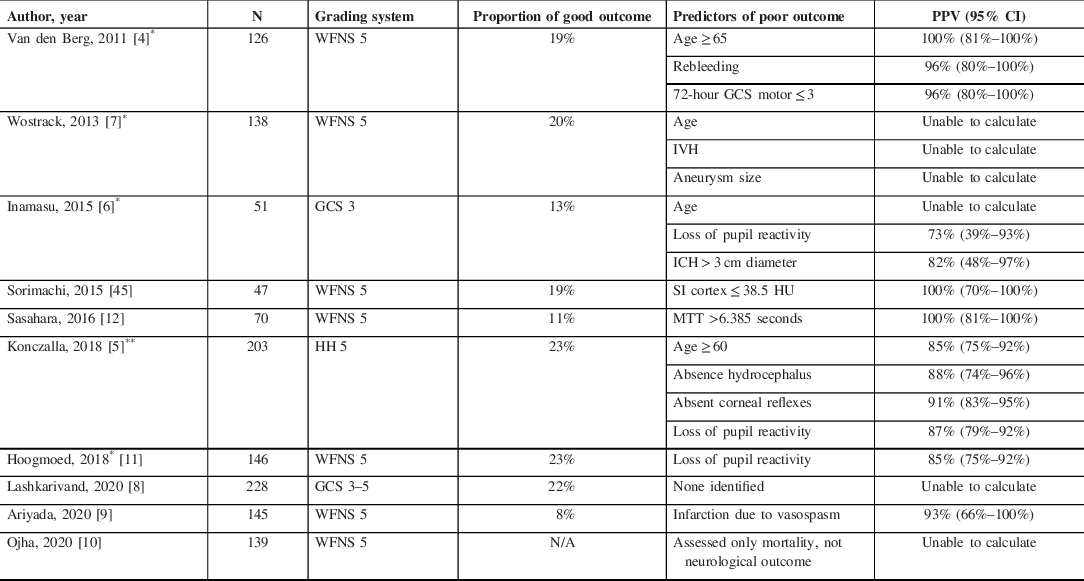
CTP = CT perfusion; DCI = delayed cerebral ischemia; GCS = Glasgow Coma Scale; HH = Hunt–Hess; HU = Hounsfield Units; IVH = intraventricular hemorrhage; MTT = mean transit time; SI = source image; WFNS = World Federation of Neurological Surgeons.
* Sensitivity and specificity assessed only in patients in whom there was no early progression to NDD or WLSM
** Variables assessed at time of admission, not subsequently
Strengths of our study include prospective collection of most data elements and a population-based design, whereby consecutive patients within a geographic region were included. Unlike previous cohort studies involving WFNS grade 5 patients, we have explicitly determined whether death was preceded by WLSM, which allowed us to explore whether prognostic variables might be confounded by end-of-life care practices. Limitations include retrospective adjudication of some variables and that outcome assessment was not always based on in-person interaction between the study team and patients. An important consideration is also that there is no consensus regarding what constitutes “sufficient” PPV in decision-making regarding WLSM; this likely varies for individual patients and families, as well as their physicians, which is why we included both 95% and 90% as thresholds. Reference Steinberg, Callaway and Arnold48
In summary, favorable outcomes are not rare among patients with grade V SAH. Definitive assessment of prognosis is generally not possible at the time of presentation to hospital, such that most patients should initially receive aggressive treatment. Several clinical and radiographic findings between admission and 72 hours appear to have high PPV for poor outcome. Future studies are needed to validate these findings, particularly among patients with these characteristics in whom life-sustaining measures are continued for longer or in countries where WLSM is unusual for cultural reasons. Reference Suarez, Martin and Bauza1,Reference Morgenstern, Zahuranec and Sanchez49,Reference Phua, Joynt and Nishimura50,Reference Oh, Park and Choi51 More data describing findings with high PPV for poor outcome are needed before more definitive consensus guidelines can be developed regarding neuroprognostication of patients with grade 5 SAH.
Conflicts of Interest
The authors do not have any conflicts of interest to report.
Author Contributions
AK conceived the study, assisted with data collection, analyzed the data, and wrote the first draft of the manuscript and revised the manuscript.
PC assisted with data collection and assisted in data analysis and revision of the manuscript.
JK assisted with data collection and assisted in data analysis and revision of the manuscript.
SR assisted with data collection and revision of the manuscript.
SD assisted with data collection and revision of the manuscript.
AM, GS, and JW assisted with data collection and revision of the manuscript.
All authors approved the final version of the manuscript.
Funding
This work was performed with in kind support from the University of Calgary, Department of Critical Care Medicine.








