Introduction
The optimal management of adult craniopharyngioma (CP) remains controversial. These rare intracranial tumors arise from remnants of Rathke’s pouch in the sellar and parasellar areas. Reference Muller1 Although benign, CP can cause significant morbidity and mortality Reference Muller1 through compression of important adjacent structures such as the hypothalamic–pituitary axis, optic apparatus, and third ventricle. CP has a bimodal distribution with peak incidence at ages 5–14 and 50–74. Reference Bunin, Surawicz, Witman, Preston-Martin, Davis and Bruner2 Data regarding the management and outcomes of adult CP is limited. Reference Dekkers, Biermasz and Smit3 Initial treatment options for CP include gross total resection (GTR) or subtotal resection (STR) with or without adjuvant or delayed radiotherapy (RT). Additional treatments with intracavitary radioisotope brachytherapy or chemotherapy, stereotactic radiosurgery (SRS), Reference Iwata, Tatewaki and Inoue4,Reference Saleem, Hashim, Rashid and Ali5 and systemic therapy have also been investigated. Reference Barkhoudarian and Laws6,Reference Lithgow, Pohl, Karavitaki, Feingold, Anawalt and Boyce7 With this multitude of options, the optimal upfront management strategy remains debatable. Reference Muller1,Reference Lithgow, Pohl, Karavitaki, Feingold, Anawalt and Boyce7–Reference Jensterle, Jazbinsek and Bosnjak9
Treatment of CP is challenging, particularly due to its high recurrence rate and morbidity associated with a total resection and with disease recurrence. Reference Lithgow, Pohl, Karavitaki, Feingold, Anawalt and Boyce7,Reference Grewal, Spielman and Safi8,Reference Steno, Bizik, Steno and Matejcik10,Reference Park, Dho, Kim, Kim, Park and Kim11 In most series, recurrences are seen, on average, in 20% of patients undergoing GTR and 60% of patients undergoing STR. Reference Lithgow, Pohl, Karavitaki, Feingold, Anawalt and Boyce7,Reference Grewal, Spielman and Safi8,Reference Becker, Kortmann, Skalej and Bamberg12 While resection helps alleviate some of the compressive symptoms caused by CP, it can also cause iatrogenic complications. GTR can result in significant morbidity, particularly pan-hypopituitarism, diabetes insipidus, hypothalamic obesity, and visual impairment. Reference Grewal, Spielman and Safi8,Reference Schoenfeld, Pekmezci and Barnes13,Reference van Iersel, Meijneke and Schouten-van Meeteren14 Although these issues may be present prior to surgery, they may worsen in 40–100% of patients undergoing resection. Reference Grewal, Spielman and Safi8,Reference Jensterle, Jazbinsek and Bosnjak9,Reference Mende, Kellner and Petersenn15 To avoid extensive sequelae from GTR, one may opt for STR followed by observation. However, this treatment strategy would carry a high risk of recurrence and need for salvage treatment. The role of RT thus far has been to decrease the risk of recurrence, particularly after incomplete resection. Reference Muller1,Reference Grewal, Spielman and Safi8,Reference Park, Dho, Kim, Kim, Park and Kim11,Reference Schoenfeld, Pekmezci and Barnes13,Reference Manaka, Teramoto and Takakura16–Reference Sadashivam, Menon, Abraham and Nair18 However, the optimal timing of RT has yet to be established, as it may be used immediately after STR or as salvage with or without preceding debulking. Reference Muller1
The purpose of this study is to present our institution’s experience with the treatment of adult CP and compare disease control outcomes based on the initial treatment strategy.
Methods
After obtaining institutional review board approval, data were collected retrospectively through chart review. All patients diagnosed with adult CP at our institution from 1999 to 2020 were identified through a pathology database search. Data collected included age at diagnosis, gender, tumor location, histologic subtype, size prior to treatment and after initial surgery, initial treatment modality and dates, RT gross tumor volume (GTV), RT dose, relapse dates and salvage treatments, last follow-up dates and imaging, secondary neoplasms, and, when available, long-term visual, endocrine, and cerebrovascular outcomes.
Twelve patients had an initial craniotomy, and the others had a transsphenoidal resection of their CP. The extent of resection was determined intraoperatively and on postoperative imaging studies. Seven patients received adjuvant RT and another nine received salvage RT. The median delivered RT dose was 54 Gy (range: 46.8–54) in 1.8–2 Gy per fraction. All patients received linear accelerator-based stereotactic intensity-modulated RT (IMRT) with 6 MV photons. The earliest RT treatment in this cohort was in 2008. Patients were simulated supine with a thermoplastic mask for immobilization. Thin-cut contrast-enhanced computed tomography (CT) and, when available, magnetic resonance imaging (MRI) simulation scans with 1–3 mm slices were done. Image registration and fusion were done with diagnostic contrast-enhanced preoperative and postoperative CT and MRI. Treatment volumes included a GTV, defined as the tumor seen on simulation scans, and a planning target volume (PTV). A 2–15 mm margin was used for PTV expansion, with 88% (14/16) ranging between 2 and 5 mm. Image-guidance RT was done using the ExacTrac system or weekly cone-beam CT. Images were monitored closely for potential tumor changes requiring cyst drainage or re-planning.
Local control was evaluated on follow-up contrast-enhanced CT and/or MRI every 6–12 months. Disease progression was defined as growth on imaging requiring further intervention. Physician follow-up included evaluations by ophthalmology, endocrinology, neurosurgery, and radiation oncology, if treated with RT. Follow-up time was measured from initial treatment to last imaging, physician or laboratory follow-up documentation, or death, whichever occurred last.
Data were collected with Microsoft Excel 2021. Statistical analyses were performed using SAS version 9.0 (SAS Institute, Cary, NC) and R (R foundation for statistical computing, Vienna, Austria). Descriptive statistics were used to assess patient characteristics. The Kaplan Meier curve was used to assess progression-free survival (PFS) and corresponding 95% confidence intervals (CI) from time since treatment initiation in the overall study population and by treatment group. Log-rank test was used to compare PFS between treatment arms up to 10 years.
Results
We identified 24 patients with adult CP (Table 1). Upon archived chart review, one patient was found to have had a GTR for a CP at an outside institution at the age of 13. All other patients were 18 years of age or older. Presenting signs and symptoms included visual deficits, behavioral or cognitive changes, headaches, and endocrine disturbances in 16, 9, 8, and 6 patients, respectively. Six tumors were abutting the optic chiasm, and nine were extending to the third ventricle.
Table 1: Patient characteristics overall and stratified by treatment

n: number; IQR: interquartile range; SD: standard deviation; GTR: gross total resection; STR: subtotal resection; RT: radiotherapy.
Initial Treatment
Seven patients had an initial GTR, 10 had an initial STR alone, and 7 had an initial STR followed by adjuvant RT. Of the GTR patients, four had a transsphenoidal surgery (TSS) while three had craniotomies. Of the STR alone patients, six had a TSS, and four had a craniotomy. Of the STR and adjuvant RT patients, two had a TSS, and five had craniotomies. Patients undergoing GTR did not receive adjuvant RT. The median residual disease volume for patients who had initial STR alone was 3.2 cm3. Patients in the STR plus adjuvant RT group-initiated RT shortly after STR (mean: 2.9 months, standard deviation: 2.5 months). The median GTV volume for patients who received RT at our institution was 6.4 cm2. Two patients did not have complete information regarding their RT treatment as they were treated at another institution. RT dose and duration were, however, available for all patients. Patients managed with initial surgery alone required a median of two surgeries for the management of their CP (range: 1–5). Patients who received adjuvant RT required no more than one surgery, meaning the only surgery required for the management of their CP was the initial resection.
Follow-up
The median follow-up was 85 months (range: 19–258). Follow-up imaging was available for all patients included in the study, with the latest imaging being at a median of 77 months after initial treatment (range: 14–255). Median follow-up since adjuvant RT was 71 months (range 15–148) and 37 months for salvage RT (range 3–111).
Outcomes
A total of 4 out of 7 patients (57%) with initial GTR and 7 out of 10 patients (70%) with initial STR had a relapse. None of the patients treated with STR followed by adjuvant RT developed disease progression or relapse. Of the 17 patients initially treated with surgery alone, 9 (53%, 3 GTR and 6 STR) underwent salvage RT due to disease progression at a median time of 46 months. There was no disease progression after salvage RT. Of the 10 patients treated with STR alone initially, 5 underwent a median of 2 additional surgeries (range: 1–3), whereas patients having undergone STR with adjuvant RT required no additional surgeries. Four patients treated with initial GTR required a median of one additional surgery (range: 1–2). None of the patients having undergone STR with immediate adjuvant RT required further resection.
The 3-year overall PFS was 73% (95% CI: 57–94%). The 3-year PFS was 100% in the STR plus RT group, 86% (95% CI: 63–100%) in the GTR group, and 47% (95% CI: 23–94%) in the STR alone group. The 5-year overall PFS was 56% (95% CI: 38–83%). The 5-year PFS was 100% in the STR plus RT group, 69% (95% CI: 40–100%) in the GTR group, and 18% (95% CI: 3–91%) in the STR alone group (Figure 1).

Figure 1: Kaplan Meier for progression-free survival stratified by treatment group with the start of follow-up corresponding to treatment initiation. GTR: gross total resection; STR: subtotal resection; RT: radiotherapy.
Log rank p-value = 0.01
Toxicity
RT was well-tolerated with only mild acute toxicity such as headaches in nine patients, fatigue in nine patients, nausea and vomiting in two patients, and ocular dryness in two patients. One patient could not complete RT due to shunt dysfunction requiring repair and stopped RT at 46.8 Gy. Endocrine disturbances were seen in 13 patients after surgery and 3 patients after RT. Four patients had no endocrine changes after treatment. Seven patients had no visual changes after treatment, five had improved vision after surgery, and two after RT. Six patients had worsening of their vision after surgery. Two patients were reported to have a cerebrovascular event (ischemic stroke) at the age of 70. One 2 years after adjuvant RT, and the other 4 years after salvage RT. The first patient had undergone multiple shunt repairs after salvage RT, while the second had a poor baseline performance status prior to treatment and had preexisting cardiac comorbidities and major cognitive disorder. No secondary neoplasm or malignant transformation was noted. There were no treatment-related deaths. Further detailed toxicity outcomes are reported in Table 2.
Table 2: Treatment toxicity outcomes
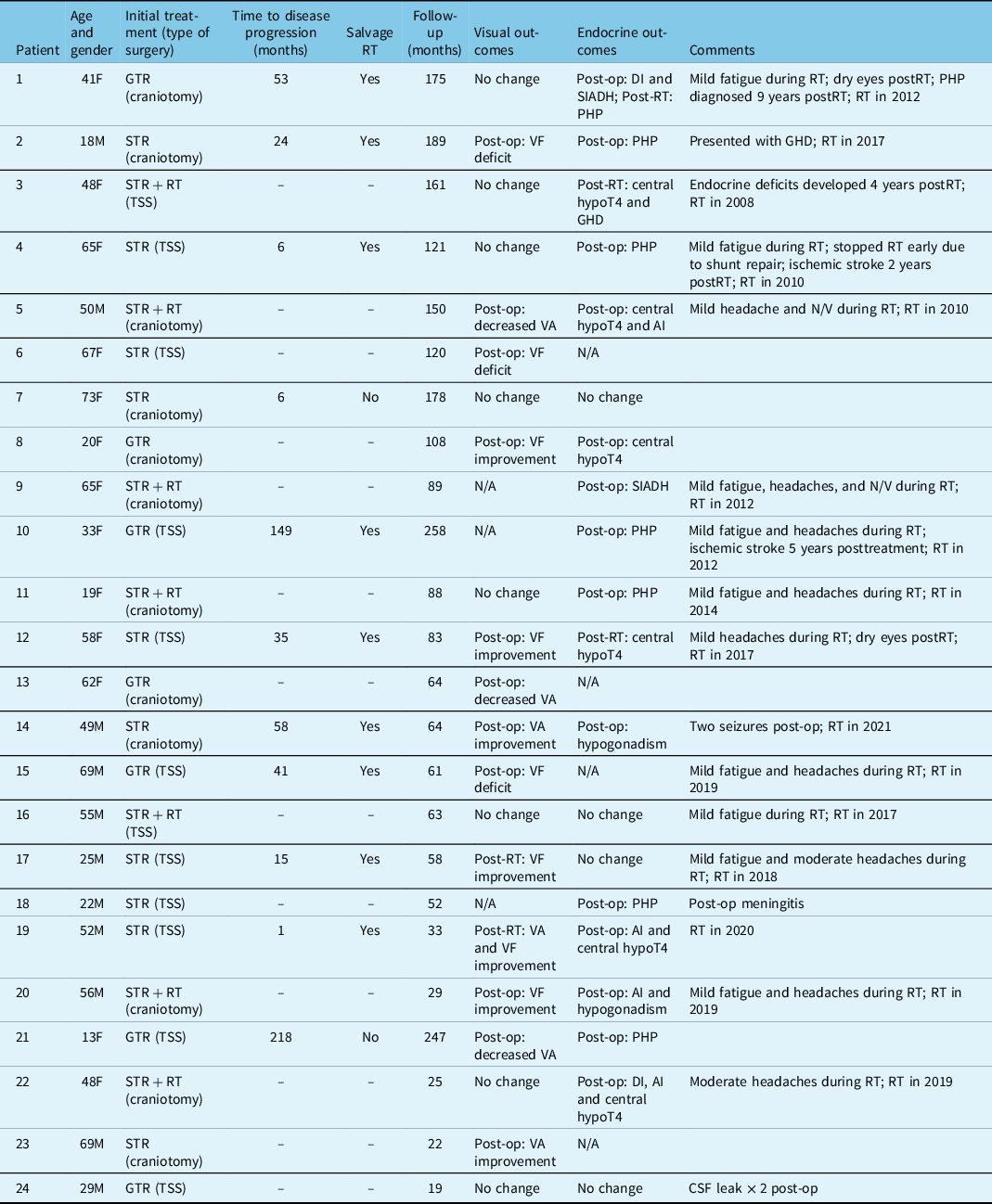
F: female; M: male; TSS: trans-sphenoidal surgery; Post-op: postoperative; DI: diabetes insipidus; SIADH: syndrome of inappropriate antidiuretic hormone secretion; RT: radiotherapy; PHP: panhypopituitarism; VF; visual field; N/V: nausea/vomiting; GHD: growth hormone deficiency; STR + RT: subtotal resection and adjuvant radiotherapy; VA: visual acuity; hypoT4: hypothyroidism; AI: adrenal insufficiency; CSF: cerebrospinal fluid; N/A: not available.
Discussion
As CP patients typically live a near-normal lifespan, Reference Hankinson, Fields and Torok19 disease control after initial treatment and the use of salvage interventions need to be balanced with iatrogenic treatment toxicity. Recurrences are seen in up to 62% of patients having undergone GTR Reference Lithgow, Pohl, Karavitaki, Feingold, Anawalt and Boyce7 and up to 75% with STR Reference Lithgow, Pohl, Karavitaki, Feingold, Anawalt and Boyce7,Reference Sadashivam, Menon, Abraham and Nair18 at 10-year follow-up. In our cohort, we observed comparable recurrence rates after surgery with 57% seen after GTR alone and 70% after STR alone. The extent of surgery has not been shown to impact on survival, Reference Hankinson, Fields and Torok19,Reference Lehrich, Goshtasbi, Hsu and Kuan20 and conflicting data exist regarding the morbidity associated with GTR versus STR. While some reports suggest no difference in long-term visual, endocrine, and hypothalamic outcomes in adults treated with STR compared to GTR, Reference Sadashivam, Menon, Abraham and Nair18,Reference Lee, Kim and Seoul21 others show increased postoperative and long-term morbidity Reference Rock, Dincer, Carr, Opalak, Workman and Broaddus22 with GTR, particularly regarding endocrine dysfunction. Reference Schoenfeld, Pekmezci and Barnes13,Reference Akinduro, Izzo and Lu23 However, with the high recurrence rates seen with STR alone, adjuvant RT has been used and shown to compensate for a more limited surgical approach by decreasing recurrence rates to that of a GTR. Reference Muller1,Reference Park, Dho, Kim, Kim, Park and Kim11,Reference Schoenfeld, Pekmezci and Barnes13,Reference Sadashivam, Menon, Abraham and Nair18
Our results suggest that RT is effective and may even offer a potential benefit over surgery alone in the management of adult CP. STR followed by adjuvant RT as a definitive management, compared to resection alone, seems to result in superior PFS and obviate the need for subsequent surgical interventions. In addition, in our experience, salvage RT resulted in no disease progression upon a median follow-up of 37 months postRT. Interestingly, adjuvant RT is underutilized in adults compared to the pediatric population. Lehrich et al. reported significantly higher rates of adjuvant RT in the pediatric population (34.3% compared to 22.3% in adults), as well as a better overall survival (OS) and a lower 90-day mortality. Reference Lehrich, Goshtasbi, Hsu and Kuan20 In our cohort, the median age of patients having undergone an initial GTR was lower than those having undergone STR (27 versus 62 and 50). However, no correlation was found between age and disease progression, and none has been reported in the literature. Reference Lehrich, Goshtasbi, Hsu and Kuan20
Morbidity from CP, regardless of treatment, remains high. Reference Muller1,Reference Rock, Dincer, Carr, Opalak, Workman and Broaddus22 In our study, all patients having received either adjuvant or salvage RT remained with their disease controlled. Toxicity profiles also did not appear to differ significantly compared to surgical management alone. In fact, endocrine dysfunction seems to be more frequently seen in GTR patients. Reference Schoenfeld, Pekmezci and Barnes13,Reference Akinduro, Izzo and Lu23 In our cohort, development or worsening of endocrine function was observed in 4/7 (57%) patients having had an initial GTR. In general, endocrine function deficits were seen more commonly in patients postresection with 13 patients (54%) developing deficits postsurgery and 3 (19%) postRT (Table 2). Schoenfeld et al. also reported similar outcomes with panhypopituitarism seen in 56% of GTR patients and 13% of STR + RT patients. Reference Erfurth, Holmer and Fjalldal24 Visual outcomes were improved in five patients postsurgery and two patients postRT. Due to risks associated with attempting GTR, some centers favor opting for a STR followed by observation. The main drawbacks of undergoing STR without immediate adjuvant RT are higher recurrence rates and need for additional surgical interventions. In our cohort, out of the 10 patients treated with STR alone, 6 recurred and all but one required a median of two additional surgeries prior to salvage RT. The risk of disease-progression-related and surgical morbidity is thus increased. After repeat resections and before salvage RT, two patients had worse visual defects, three had worse endocrine deficits, and one developed seizures. Four patients treated initially with GTR alone required additional surgery, with two of them developing worsened visual outcomes after re-resection. As in our study, disease control after adjuvant versus salvage RT remains similar. Reference Moon, Kim and Park25,Reference Pemberton, Dougal, Magee and Gattamaneni26 Where adjuvant RT may be better than salvage is in regards to morbidity. As seen in our cohort, patients recurring after STR often undergo additional surgeries and may develop worsening symptoms after relapse. Although disease control appears to be similar with adjuvant versus salvage RT, additional surgeries prior to RT may result in worse progression-free survival. Reference Masson-Cote, Masucci and Atenafu27 Literature regarding the quality of life and toxicity from adjuvant versus salvage RT is limited. Moon et al. Reference Moon, Kim and Park25 looked specifically at this issue and found that visual field and acuity were better with adjuvant RT, as well as improvement of diabetes insipidus. Interestingly, this is similar to our findings with increased visual morbidity after relapse. Pemberton et al., Reference Pemberton, Dougal, Magee and Gattamaneni26 on the other hand, did not find any significant difference in the quality of life comparing immediate RT to salvage. Lastly, although not seen in the adult population, Regine et al. Reference Regine, Mohiuddin and Kramer28 reported worse survival outcomes in the pediatric population when RT was given upon recurrence versus upon initial treatment. The decision to favor adjuvant versus delayed RT remains to be elucidated by larger prospective studies. This being said, most patients having undergone STR alone will eventually recur, and the main benefit of adjuvant RT is to avoid worsening of morbidity from disease progression and/or re-resection. Ultimately, decision regarding adoption of one strategy versus the other remains to be made with the patient and multidisciplinary team. The main concerning long-term RT-related toxicities are cognitive deterioration, cerebrovascular events, and secondary tumors. Reference Masson-Cote, Masucci and Atenafu27 In our cohort, two cerebrovascular events were noted, and there were no secondary neoplasms. With increasingly precise RT allowing for smaller treatment volumes and sparing of surrounding structures, these risks are lowered. Reference Aggarwal, Fersht and Brada29,Reference Conti, Pontoriero and Ghetti30 It is also difficult to differentiate effects of tumor growth, hormonal treatment, surgical procedures, and RT on cognitive changes, Reference Erfurth, Holmer and Fjalldal24 as deterioration is often multifactorial. Direct comparison of surgery, RT, and comorbidities due to endocrinopathies in regard to the risk of cerebrovascular events is also challenging, Reference Erfurth, Holmer and Fjalldal24 and no evidence exists to this date showing superiority of surgery versus RT. Disease control outcomes comparing GTR to STR with adjuvant RT are also conflicting. While some show improved control with GTR at the cost of increased toxicity, Reference Akinduro, Izzo and Lu23 others show similar or better clinical outcomes with STR and adjuvant RT. Reference Park, Dho, Kim, Kim, Park and Kim11,Reference Schoenfeld, Pekmezci and Barnes13,Reference Wang, Zhang, Feng and Guo31–Reference Zhang, Verma and Lyden33 Furthermore, adjuvant RT has no deleterious impact on overall survival Reference Wang, Zhang, Feng and Guo31 and may in fact provide a survival advantage over surgery alone. Reference Manaka, Teramoto and Takakura16,Reference Hankinson, Fields and Torok19 These findings point towards an overall superiority of STR followed by adjuvant RT for the initial management of adult CP.
In a population of patients with a long survival, minimizing the need for subsequent surgeries is important. Quality of life is significantly deterred with repeated surgery. Reference Dekkers, Biermasz and Smit3,Reference Jensterle, Jazbinsek and Bosnjak9,Reference van Iersel, Meijneke and Schouten-van Meeteren14,Reference Mende, Kellner and Petersenn15,Reference Gautier, Godbout and Grosheny34 Surgeries for recurrences also carry a higher perioperative mortality rate and lower overall survival. Reference Jose, Rajan, Ashley, Marsh and Brada17,Reference Karavitaki, Brufani and Warner35 As patients in our cohort did not recur after the introduction of RT in their management, they required fewer interventions after RT. Twelve out of sixteen (75%) patients who had either adjuvant or salvage RT received 54 Gy in 1.8 Gy per fraction. Despite small numbers, there was no difference between those receiving 54 Gy versus less in terms of disease control. This raises the question of whether dose de-escalation may be done with similar results and potentially fewer toxicities. In fact, Combs et al. showed excellent 5- and 10-year PFS of 100% with a median dose of 52.2 Gy in conventional fractionation. Reference Combs, Thilmann, Huber, Hoess, Debus and Schulz-Ertner36 Definitive RT in the setting of adult CP has also been investigated. Reference Iwata, Tatewaki and Inoue4,Reference Conti, Pontoriero and Ghetti30,Reference Zhang, Verma and Lyden33,Reference Takano, Akutsu, Mizumoto, Yamamoto, Tsuboi and Matsumura37 Zhang et al. found no difference in outcomes between definitive RT, GTR, and STR with adjuvant RT Reference Zhang, Verma and Lyden33 in 1218 patients included in the Surveillance Epidemiology and End Results database from 2004 to 2012. Hypofractionated adjuvant RT and SRS have also shown promise with high tumor control rates, low toxicity, and shorter overall treatment time, Reference Iwata, Tatewaki and Inoue4,Reference Conti, Pontoriero and Ghetti30 and warrant further outcome and toxicity comparison with normofractionated RT. As adult CP are rare and benign tumors with heterogeneous behavior requiring a multidisciplinary approach, large randomized studies comparing surgery alone to partial surgery plus RT is nearly impossible. Lastly, the discovery of BRAF mutation in up to 95% of papillary CP has led to the use of targeted therapies in this histologic type, Reference Holsken, Sill and Merkle38 which shows great promise. Although more common in the adult population, papillary CP constitutes 5–30% of adult CP. Reference Momin, Recinos and Cioffi39 The adamantinomatous type is more common and constitutes most of our study population (22/24). This type harbors CTNNB1 or APC mutations encoding for beta-catenin, which are being investigated for potential targeted therapy. Reference Whelan, Hengartner, Folzenlogen, Prince and Hankinson40,Reference Hengartner, Prince, Vijmasi and Hankinson41
This study is limited by its relatively small number of patients and its retrospective nature with its inherent lack of standardized, centralized, and complete documentation of long-term side effects of treatment such as quality of life measures and proper cognitive assessments. Although a p-value of 0.01 is given in Figure 1, it is only assessing whether there is a statistically significant treatment difference between any of the groups without telling us which groups are statistically different. A more useful comparison would be pairwise between each of the treatment groups, but we were underpowered for that type of analysis. A major strength of the study is the long-term clinical and imaging follow-up. We also confirmed better outcomes with STR and adjuvant RT compared to GTR alone. This is an important finding that warrants further validation with larger studies.
Conclusion
Our experience in the treatment of adult CP suggests that adjuvant and salvage RT is effective in the management of these tumors, and that STR plus RT may be associated with improved PFS and less toxicity compared to GTR. Larger studies directed towards rigorous and prospectively collected outcomes are needed to corroborate these findings.
Acknowledgments
The authors wish to thank all collaborators at their institution.
Funding
The current study did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Disclosures
The authors have no disclosures.
Statement of Authorship
Conceptualization and design of study: JK, LS, VPR. Acquisition of data: JK, SK, MT, MCG, BA, GS, LS, VPR, DS. Analysis and interpretation of data: JK, FKK. Writing – original draft preparation: JK. Writing – review and editing: GS, LS, VPR, FKK. All authors have read and agreed to the submitted version of the manuscript.





