Highlights
-
Five patients with refractory trigeminal pain underwent epidural motor cortex stimulation without complications.
-
All patients achieved >50% pain relief at 12 months (mean reduction: 77 ± 13%).
-
The outcomes exceed literature averages, highlighting the necessity of larger, multicentric trials to evaluate the efficacy of motor cortex stimulation for painful trigeminal neuropathy.
Introduction
Neuromodulation is garnering ever-increasing interest as a therapeutic option for refractory cases of trigeminal pain. In their pursuit of a novel therapeutic approach to address intractable pain syndromes, Tsubokawa and his colleagues empirically stimulated various brain areas in animal models of neuropathic pain. They discovered that stimulation of the primary motor cortex provided pain relief, reducing thalamic hyperactivity and pain-related behavior in cats with spinothalamic transections. In accord with these experimental results, their ensuing clinical application yielded satisfactory pain relief in patients afflicted with intractable central pain syndromes. Reference Tsubokawa, Katayama, Yamamoto, Hirayama and Koyama1–Reference Tsubokawa, Katayama, Yamamoto, Hirayama and Koyama3 Since its inception in the early 1990s, motor cortex stimulation (MCS) has been shown to be a safe and effective neuromodulation therapy for trigeminal neuralgia (TN) and painful trigeminal neuropathy (PTN), Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4,Reference Mo, Hu, Zhang, Wang, Liu and Zhao5 despite its current off-label status in Canada. Nonetheless, a rich history of heterogeneous results Reference Ebel, Rust, Tronnier, Böker and Kunze6–Reference Carroll, Joint, Maartens, Shlugman, Stein and Aziz10 has fostered skepticism regarding the long-term effectiveness of MCS. Indeed, outcomes vary widely, with reported success rates ranging from 12.5% to 100% across different studies. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 While the precise mechanism of action of MCS remains elusive, three decades of research suggest the activation of two primary, synergistic mechanisms: modulation of the sensory-discriminative component of pain through descending inhibitory pathways and modulation of the motivational-affective component of pain. Reference Garcia-Larrea and Peyron11–Reference Bliss, Collingridge, Kaang and Zhuo14
PTN is characterized by continuous facial pain in the trigeminal nerve distribution, and associated somatosensory signs and symptoms are common. Superimposed paroxysms of pain may occur but are not the predominant pain type, a key difference with TN. 15 The treatment of PTN is challenging due to its poor response rate to medical treatment and its complexity arising from PTN’s multiple etiologies and sub-classifications. Reference Benoliel, Teich and Eliav16–Reference Fornasari18 Current medical management for PTN includes antiepileptic medications, tricyclic antidepressants, and serotonin-noradrenaline reuptake inhibitors, with some patients additionally requiring strong opioids. Reference Fornasari18 Patients with PTN do not derive the same benefit from surgical and neuroablative procedures as those with TN, as further injury to an already compromised nerve can exacerbate neuropathic pain. Reference Burchiel and Raslan19 In the absence of established surgical or interventional therapies, neuromodulation emerges as a promising alternative, providing a critical avenue of hope for patients enduring chronic trigeminal facial pain. Various neuromodulatory interventions, such as peripheral nerve stimulation, Reference Ni, Yang, Han, Guo, Huang and Weng20 deep brain stimulation Reference Boccard, Pereira, Moir, Aziz and Green21,Reference Frizon, Yamamoto, Nagel, Simonson, Hogue and Machado22 and MCS, Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4,Reference Mo, Hu, Zhang, Wang, Liu and Zhao5 have been employed in the management of chronic orofacial pain. Although meta-analyses have assessed the efficacy of these approaches across a range of pain conditions, data specific to PTN remain scarce. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4,Reference Mo, Hu, Zhang, Wang, Liu and Zhao5,Reference Ni, Yang, Han, Guo, Huang and Weng20,Reference Frizon, Yamamoto, Nagel, Simonson, Hogue and Machado22 This is compounded by limitations such as small sample sizes and heterogeneous study populations, which often yield inconsistent results across studies.
A recent meta-analysis reviewed MCS outcomes from 23 studies (mean sample size: 6), encompassing over 140 patients with orofacial pain of various etiologies, including TN and PTN. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 Despite this effort, a paucity of data persists for individual etiologies, alongside significant variability in outcomes and success rates across these conditions. The average pain reduction achieved with MCS ranged from 20–40% for conditions such as dental avulsion pain (n = 8), post-surgical pain (n = 11), and atypical facial pain syndromes (n = 4) to 70%–90% for conditions like anesthesia dolorosa (n = 11) and post-herpetic neuralgia (n = 8). Furthermore, these findings are aggregated from studies employing heterogeneous MCS methodologies (e.g., variations in lead placement and stimulation parameters), further complicating efforts to establish definitive efficacy rates for specific etiologies. This underscores the need to contribute additional case reports to the literature to refine these estimates and ultimately optimize the use of MCS for trigeminal pain syndromes in which it demonstrates the highest efficacy.
This article presents a case series of five patients from the Atlantic provinces of Canada with refractory PTN unresponsive to medical and surgical interventions, who achieved significant pain relief through transcranial epidural MCS.
Our mixed cohort comprises patients with etiologies associated with favorable outcomes in the literature (e.g., post-herpetic neuralgia) and those with relatively poor outcomes (e.g., dental avulsion pain). We aim to quantify the degree of pain relief and opioid reduction achieved with MCS in this cohort, provide a detailed account of the MCS technique and offer insights into its off-label application in Canada. Notably, this is only the second Canadian study to explore MCS as a treatment for trigeminal pain. Ultimately, we aim to contribute these cases to the literature to better our understanding of MCS efficacy for trigeminal pain syndromes of various etiologies.
Methods
Patient selection and data collection
The population targeted in this study included patients with persistent trigeminal pain from the Atlantic provinces who had been selected to undergo MCS surgery at the Moncton Hospital (Horizon Health Network) between July 2020 and January 2023. We framed this study as a retrospective cohort analysis. The inclusion criteria were a diagnosis of refractory trigeminal pain with maximal medical/surgical therapy and the absence of other treatment options for the patients. Exclusion criteria were as follows: age > 70 years; duration of pain progression < 1 year; no previous medical treatment; suspicion of substance abuse disorders; mild or major cognitive impairment; severe psychiatric illness, including severe depression, psychosis and suicidal ideation; medical diagnoses including active cancer; cancer in remission for < 5 years; severe cardiac disease; previous stroke or repetitive TIAs; uncontrolled diabetes mellitus (HbA1c > 8.0%); autoimmune disease requiring disease-modifying antirheumatic drugs; multiple sclerosis. Patients were followed by our multidisciplinary pain team, followed by the same neurosurgeon, a pain medicine specialist, and benefited from a neuropsychological evaluation prior to study inclusion. We performed a chart review of the patients from the preoperative period to at least 12 months of postoperative follow-ups.
Surgical intervention
The surgical intervention consisted of an awake craniotomy with local anesthesia using a circumferential scalp block technique. A preoperative navigation MRI with tractography of the corticospinal tract was used to map the motor cortex (Figure 1), minimizing the extent of the linear retro-coronal surgical incision and centralizing the craniotomy flap over the motor cortex, with extension to the sensorimotor and premotor areas. Neuronavigation with the StealthStation™ S7 system (Medtronic, Minneapolis, MN, USA) was used for the first three cases, and the StealthStation™ S8 system was used for the last two, with anatomical and diffusion tensor imaging mapping. Once the dura was exposed, a Nicolet® biphasic cortical stimulator (Natus Medical Incorporated, Middleton, WI, USA) with a constant current of 5–10 mA, pulse duration of 1 ms, and frequency of 50 Hz was used to map and identify the motor cortex, by creating facial muscle contractions, thumb and hand contractions, and, if the procedure was done on the dominant hemisphere, a speech arrest was reproduced. Once the facial representation at the motor cortex was identified, a Medtronic 16-contact surgical paddle (5-6-5) was epidurally placed with the central contact over the mapped facial motor cortex. The anterior contacts were placed over the pars opercularis. Impedances were checked, and a second mapping was done using the paddle contact and the temporary pulse generator to confirm the placement. Once the placement was confirmed, the paddle was sutured in 4 points to the underlying dura using 4–0 Prolene suture. The wires were then passed through the burr hole of the craniotomy flap, and the bone flap was repositioned using mini plates and screws (Figure 2). Two 8-contact extension leads were used and externalized through a retro-auricular counter incision and connected to the temporary external pulse generator. The scalp was then closed in the usual fashion, dressings were applied and the external pulse generator was taped to the infraclavicular area.
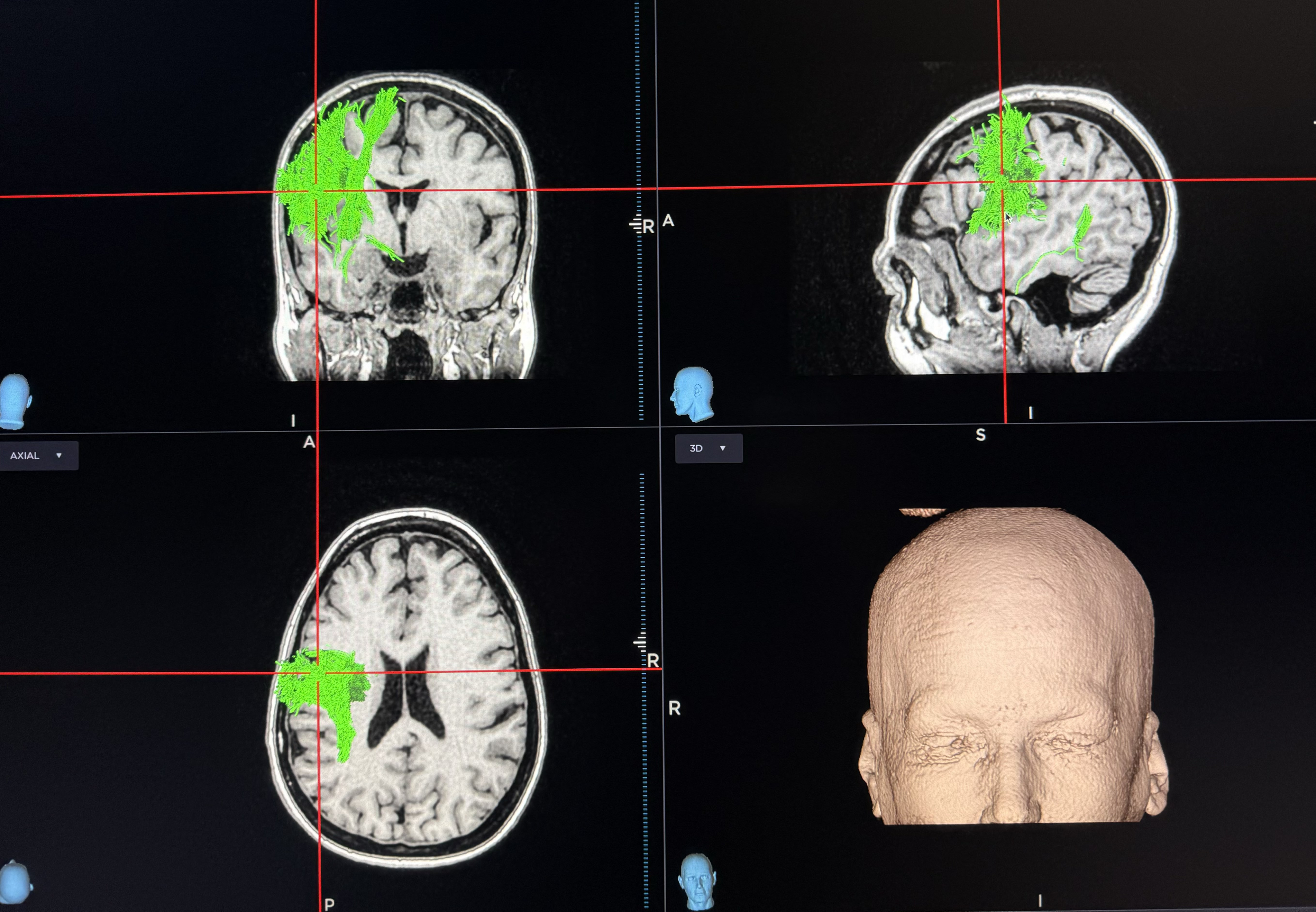
Figure 1. 3D reconstruction and tractography of the corticospinal tract, with the red marker indicating the mapped facial area during awake craniotomy.
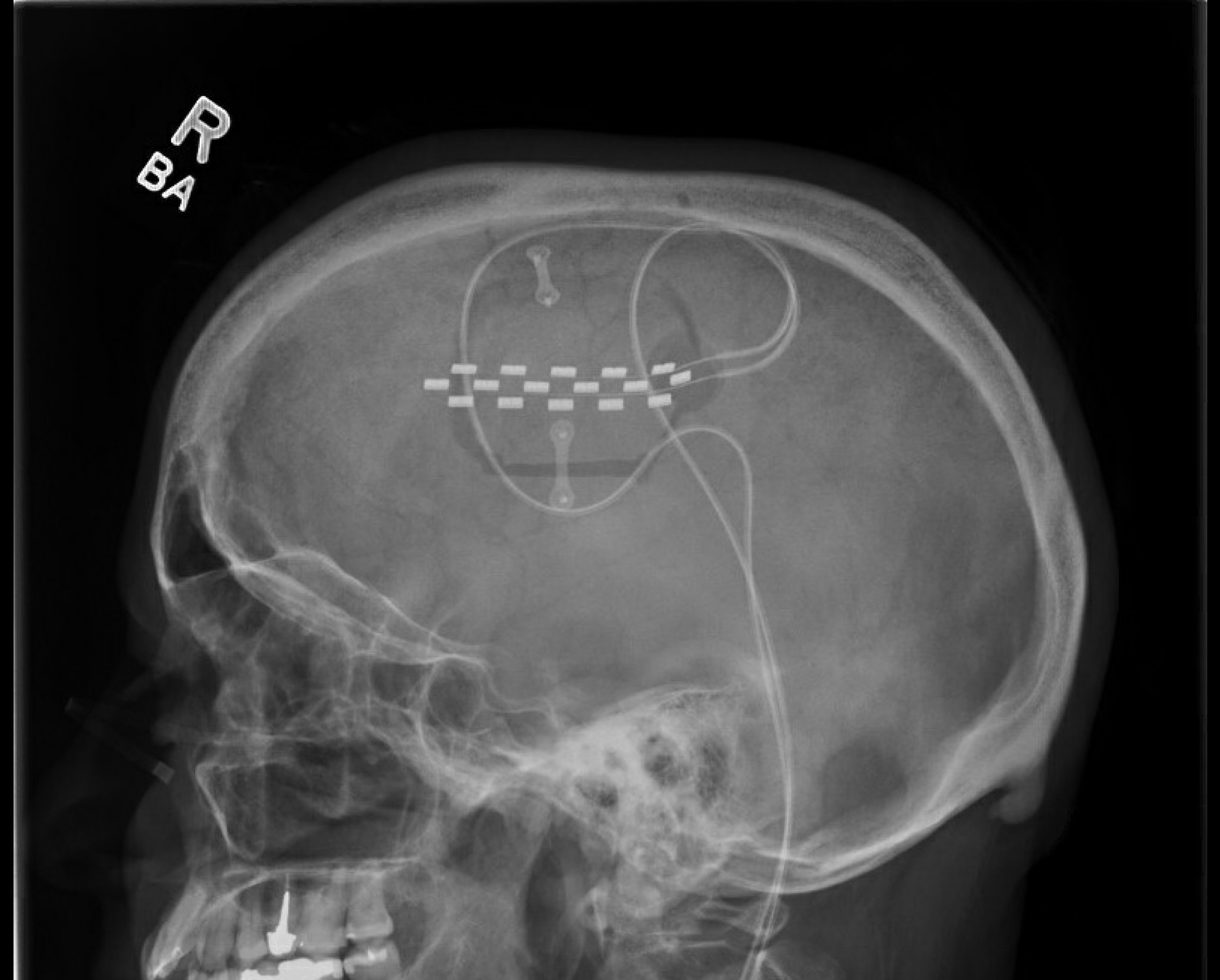
Figure 2. Lateral postoperative skull radiography demonstrating the craniotomy and the 16-contact paddle centered over the mapped motor cortex.
Postoperative course and MCS programming
Patients were admitted to the neurosurgery ward postoperatively for 24 hours. Programming was initiated three hours after surgery The anode contact was made to be the most central contact of the lead, with an additional one or two layers of cathode in a circular fashion. The initial stimulation parameters used were a pulse width of 400 ms, 50 Hz for frequency and a variable amplitude between 2 mA and 12 mA (Figure 3). The maximum amplitude was determined during the post-mapping period and was adjusted to be below the threshold of facial motor contraction, paresthesia, seizure or speech disturbance. A cycling stimulation with 10 minutes on and 10 minutes off was used, with 4-second start.
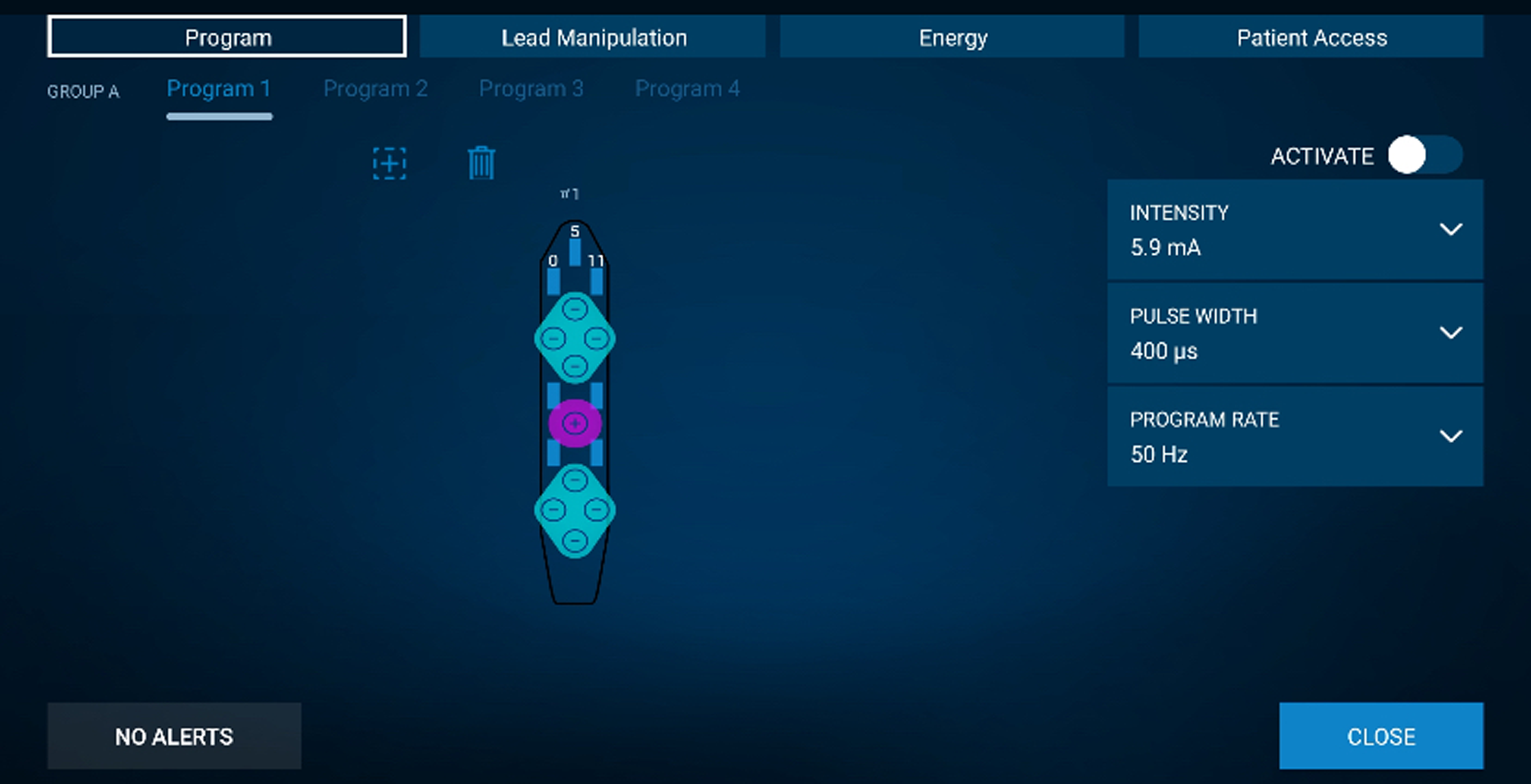
Figure 3. Medtronic surgical paddle programming with the initial trial parameters to the right. The central anode is centered over the surgically mapped motor cortex and the surrounding cathodes contact over the premotor and sensorimotor areas.
MCS trial and internalization
Patients were discharged on postoperative day one after a cerebral CT scan, and a second assessment by the neuromodulation team was performed. Efficacy was assessed in all patients with a 14-day MCS trial at home. Patients were asked to note their daily Visual Analogue Scale (VAS) in the morning and evening, their analgesic consumption, sleeping habits and activities, along with any flare-ups of their pain. A successful trial period was defined as a >50% pain reduction, with no increase in narcotic consumption and no major side effects from the stimulation. Following a successful trial, the pulse generator was internalized under general anesthesia. This procedure consisted of the removal of the external pulse generator and the extensions and tunneling of the paddle wires to an infraclavicular implantable pulse generator pocket. Four out of five participants were internalized with an Intellis™ pulse generator (Medtronic, Minneapolis, MN, USA), and one was implanted with a Vanta™ pulse generator (Medtronic, Minneapolis, MN, USA). Patients were discharged 6 hours after the internalization procedure. The amplitude could be modified by the patients between 2 mA and the previously determined maximal stimulation intensity. Possible side effects including facial muscle contractions and speech disorders ranging from mild dysarthria to aphasia were disclosed to the patients, and they were advised to reduce the amplitude in the event of adverse effects. To promote satisfactory pain relief, the pulse width could be modified from 400 to 700 ms and the frequency from 50 to 60 Hz by the neuromodulation team at follow-up appointments.
Pain medication management
During the trial period, the patient’s opioid medication was prescribed at their regular dose, and patients could reduce their daily opioid intake by 30% during the trial period. Following their wound checkup appointment at 3 weeks post-implantation, opioid medications were progressively reduced by 10% per week over a period of up to 3 months. If narcotics were stopped or decreased by more than 50% at 3 months, progressive weaning of antiepileptic or antidepressant agents was initiated, one agent at a time, following the general guidelines for each medication. If no exacerbations were observed after 12 months of stimulation, all medications were discontinued. If a patient had an exacerbation, one antiepileptic agent was kept at the minimal efficient dose.
Outcomes
The primary outcome was a 50% reduction in pain as assessed by a 10-point VAS at 12-month follow-up, a measure commonly utilized in the literature. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 Precisely, we measured the reduction in the constant component of the participant’s facial pain. A reduction in morphine equivalent dose per day (MED, mg/day) ranging from 50% to 100% has been used as a metric in various trials to evaluate the efficacy of interventions in reducing opioid consumption. Reference Eccleston, Fisher, Thomas, Hearn, Derry and Stannard23 A reduction in MED per day of 60% was defined as a secondary outcome. We reviewed changes in non-opioid medication regimes, eating habits and return to work following MCS surgery as additional outcomes.
Analysis
We used Microsoft Excel to record numerical data from patient charts and calculate aggregate statistics. Given the small sample size of our cohort, we opted to use the median and range to characterize the distribution of patient age and diagnosis/follow-up time within our cohort, as these variables had considerable variance and were prone to outliers. We used the mean and standard deviation as aggregate statistics for VAS scores and MED per day, as these variables were limited to a tighter range and were less prone to outliers despite the small sample size. Throughout the paper, the ± symbol refers to standard deviation. Figures were generated using the Python programming language (https://www.python.org/) and the NumPy (https://numpy.org/), Pandas (https://pandas.pydata.org/), Matplotlib (https://matplotlib.org/) and Seaborn (https://seaborn.pydata.org/) libraries. We did not perform any statistical tests involving p-values, given the small sample size and heterogeneity of our cohort.
Results
Patient characteristics
We included five patients who underwent surgery between July 2020 and January 2023 (Table 1, Figure 4). Three patients were excluded during the initial evaluation. Two patients were excluded for severe psychiatric illness, and one for severe heart failure, which required cardiac intervention (Figure 4). Before surgery, all patients were on a multidrug regimen for pain, including narcotics. (Table 1). The median age of the cohort was 55 (range: 30–62), and four patients (80%) were female (Table 1). Two patients were initially diagnosed with TN secondary to microvascular conflict and subsequently developed post-traumatic PTN following microvascular decompression (MVD). Two other patients had developed post-traumatic PTN following dental avulsion procedures, one of whom had MVD for concomitant microvascular conflict. The last patient was initially diagnosed with PTN attributed to herpes zoster. This patient also had MVD for concomitant microvascular conflict (Table 1). Reference Garcia-Larrea and Peyron11 All patients had constant pain with frequent pain paroxysms, somatosensory signs and symptoms in the trigeminal distribution, including hypesthesia, hyperalgesia, and mechanical and thermal allodynia. Two patients had severe weight loss and malnutrition due to an inability to eat, one requiring parenteral nutrition. Three patients exclusively ate soft foods to avoid triggering paroxysms of pain. All patients were unable to work before the operation. The mean preoperative pain level measured by a VAS was 8.4 ± 1.0 (standard deviation; SD). All patients had received maximal medical therapy and required narcotics for pain control, with a mean MED per day of 83 ± 60 (SD) mg/day. All patients in the case series were followed for at least 12 months at the time of writing (range 12–40 months).
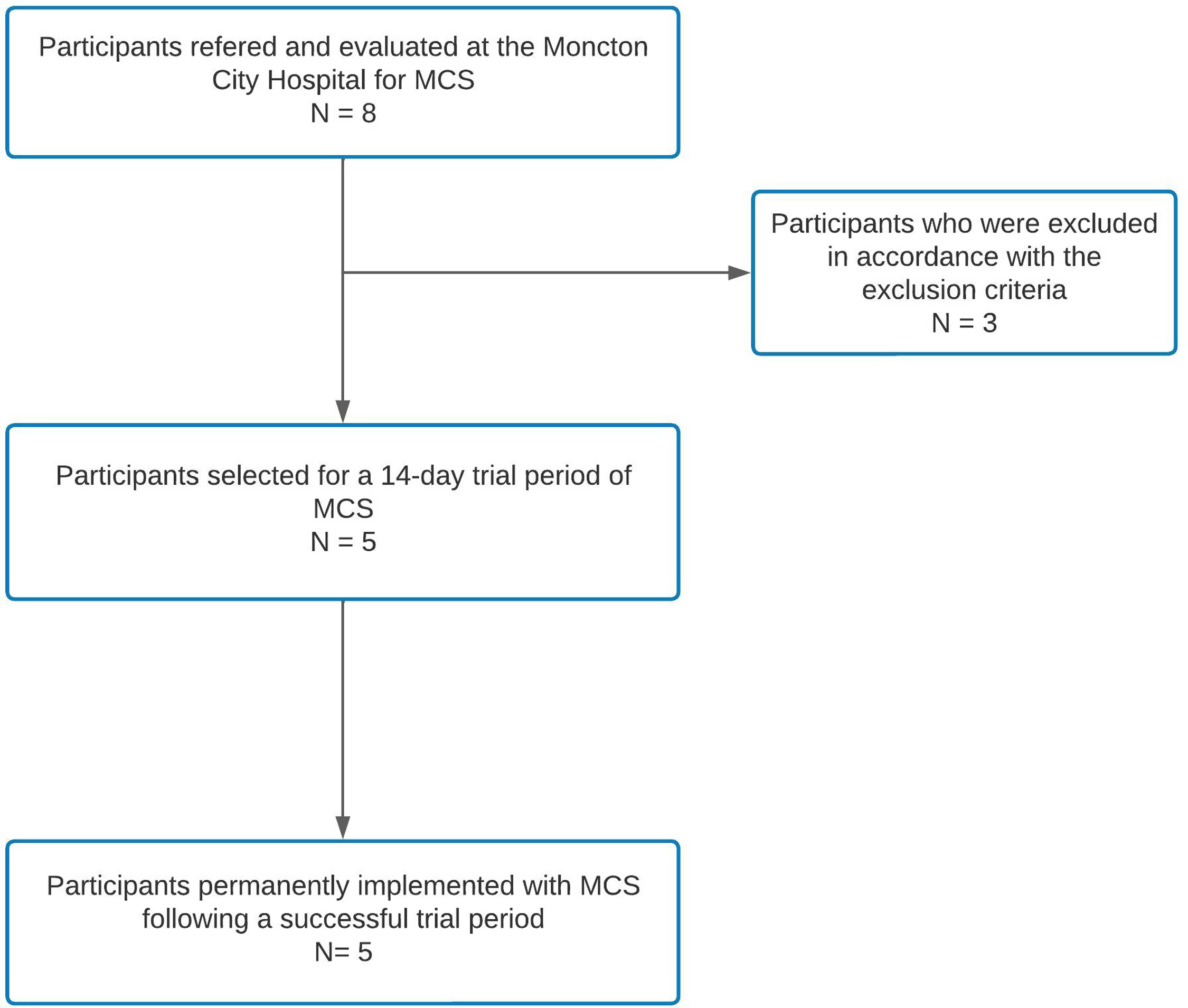
Figure 4. Flowchart depicting patient selection for motor cortex stimulation at the Moncton City Hospital.
Table 1. Individual and aggregate patient characteristics and clinical outcomes
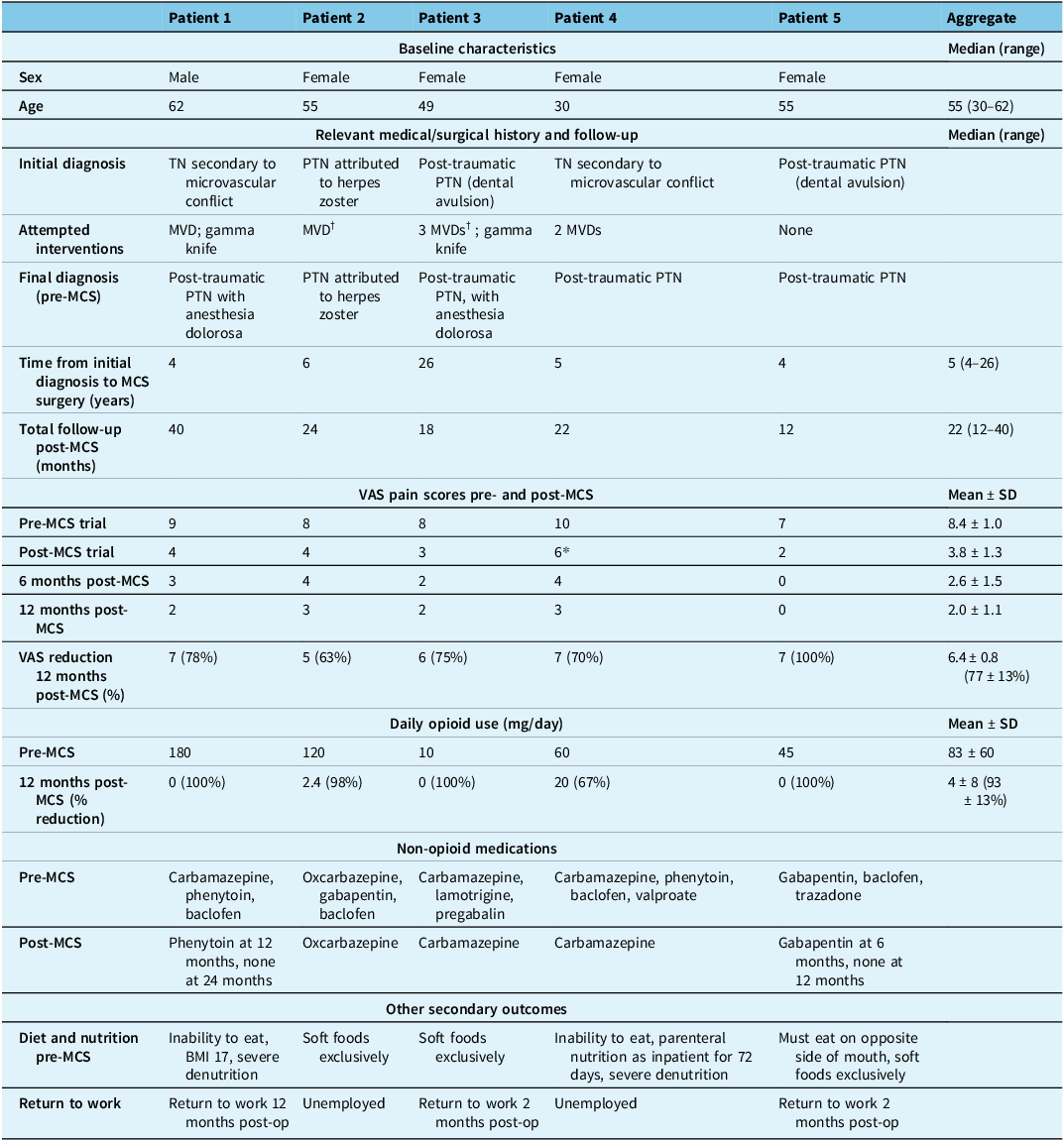
Note: TN = trigeminal neuralgia; PTN = painful trigeminal neuropathy; MVD = microvascular decompression; MCS = motor cortex stimulation.
† indicates that MVD was attempted for a concomitant neurovascular conflict.
MCS implantation and trial
All five patients underwent successful MCS implantation using the techniques described above. In four patients, battery internalization was completed following a successful 14-day trial period (pain reduction >50%). Patient 4 did not meet the successful MCS trial criteria (40% pain reduction). However, the patient and neurosurgeon collaboratively decided to proceed with internalization as the MCS trial allowed the patient to transition from a parenteral diet to a liquid diet and ultimately enabled their discharge from the hospital.
Outcomes
All five patients achieved the primary outcome of >50% pain score (VAS) reduction at 12 months (Table 1; Figure 5). The mean VAS score was reduced from 8.4 ± 1.0 (SD) preoperatively to 2.0 ± 1.1 at 12 months post-MCS. The mean VAS reduction at this time was 6.4 ± 0.8, corresponding to an average pain relief of 77 ± 13%. All five patients similarly achieved >50% pain score reduction at 6 months. Pain scores improved marginally from 6 to 12 months in three patients, while pain scores for the other two patients remained constant (Figure 5). One patient in our cohort achieved 100% pain relief 12 months post-MCS (patient 5). This patient was treated for post-traumatic PTN secondary to a dental avulsion procedure and was the only patient who had no prior surgical interventions. Patient 2 achieved the least pain relief (63%) and was the only patient in our cohort with PTN attributable to herpes zoster.
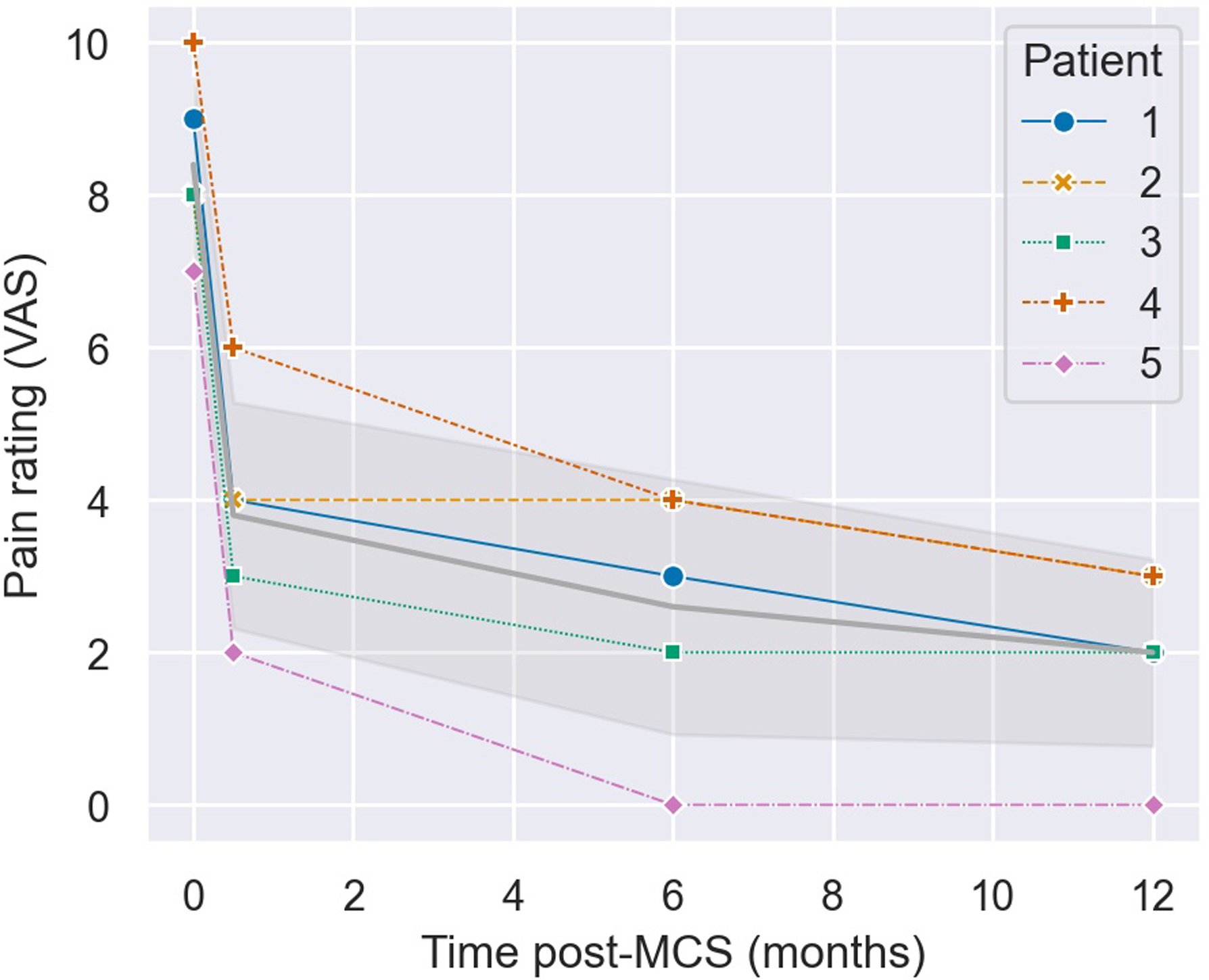
Figure 5. Evolution of pain rating scores (VAS) from preoperative assessment (time 0) to months post-motor cortex stimulation (MCS). The time point at 0.5 months represents pain ratings after the 14-day trial MCS period. The gray line with shaded error bands represents the mean ± standard deviation.
All five patients achieved the secondary outcome of a > 60% decrease in narcotic consumption as assessed by morphine equivalent dose (MED) per day. The preoperative MED per day was 83 ± 60 mg (SD), which decreased to 4 ± 8 mg/day 12 months postoperatively, resulting in an average 79 ± 62 mg/day reduction in narcotic use (93 ± 13% average consumption decrease). Three out of five patients (60%) achieved complete narcotic cessation by the 12-month follow-up. Four out of five patients (80%) achieved this goal at 18 months, and the remaining patient is continuing to taper their opioid regimen at the time of writing. Notably, Patient 1 is completely pain and medication-free at their 36-month follow-up, demonstrating a sustained and longitudinal enhancement of pain relief with MCS.
Before surgery, all patients were using multiple anticonvulsants and non-opioid analgesics with poor symptom control. Postoperatively, all patients successfully tapered off all but one non-opioid pain medication (Table 1). Two patients with severe malnutrition and weight loss achieved a successful restoration of healthy eating habits and weight recovery following the MCS procedure. Before the operation, all patients encountered difficulties with chewy foods, a concern that was effectively addressed with MCS. Furthermore, three patients successfully re-entered the workforce, with two individuals returning to work within 2 months of the operation and one patient resuming work after a year. At their most recent follow-up, all patients were satisfied with their post-surgical outcomes. We noticed stability of the pain relief after 12 months, with the same pulse width and frequency. All patients required an increase in stimulationamplitude after one year, with an average of 5.6 mA.
Safety
There were no immediate or delayed complications related to the MCS procedure. One patient implanted with a Vanta™ pulse generator had a battery depletion at 12 months, prompting a change to an Intellis™. The depletion was attributed to consistently high energy consumption, although the average recharge interval was 3.4 days.
Discussion
Summary of main results in context
This case series supports the efficacy and safety of epidural MCS as a last-resort intervention for chronic neuropathic pain. Despite some skepticism surrounding MCS in the literature – largely stemming from variable outcomes reported over the past 30 years Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4–Reference Carroll, Joint, Maartens, Shlugman, Stein and Aziz10 – most studies focusing on PTN-like syndromes have demonstrated sustained postoperative pain relief at 12 months or beyond. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4,Reference Mo, Hu, Zhang, Wang, Liu and Zhao5 However, variability in the proportion of patients achieving significant pain control and the average degree of pain reduction remains, likely reflecting the challenges posed by small sample sizes (average n = 6 in a recent meta-analysis) Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 and diverse patient populations encompassing orofacial pain syndromes of varying etiologies, further emphasizing the need for additional data. Our case series seeks to supplement the existing literature by providing additional evidence for the sustained efficacy of MCS.
All five patients in our case series achieved the primary outcome of a 50% reduction in pain (VAS) 12 months postoperatively, with a mean VAS reduction of 6.4 ± 0.8 (77 ± 13%). The results of this case series are generally more favorable than the existing literature. In recent systematic reviews and meta-analyses, patients with PTN typically experienced a mean Visual Analog Scale (VAS) reduction of 55.4% Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 and 46.5%. Reference Mo, Hu, Zhang, Wang, Liu and Zhao5 When specifying by etiology, patients with post-herpetic neuropathic pain (n = 8) had a mean pain reduction of 86.7% in the literature, Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 compared to 62.5% (n = 1) in our study. Patients with a diagnosis of anesthesia dolorosa (n = 11) and trigeminal pain from a dental avulsion (n = 8) had a mean pain relief of 73.7% and 34.4%, respectively, in the literature. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 In this case series, mean pain relief for patients with anesthesia dolorosa (n = 2) and trigeminal pain from dental avulsion procedures (n = 2) was 76 ± 1% and 88 ± 13%, respectively. While previous research has identified anesthesia dolorosa as a positive prognostic factor for MCS response, Reference Buchanan, Darrow, Monsivais, Nadasdy and Gjini24 contrasting evidence has proposed that previous neuroablative procedures were a negative response factor. Reference Raslan, Nasseri, Bahgat, Abdu and Burchiel25 This case series highlights the effectiveness of MCS for anesthesia dolorosa, irrespective of past neuroablative or neurosurgical procedures. We propose that the combination of awake craniotomy with MRI tractography and cortical mapping, alongside an MCS trial period, has contributed to the favorable outcomes observed in our case series. However, as with any study involving a small sample size, our results should be interpreted within this context, and caution should be exercised when generalizing to external cohorts, particularly given the etiological heterogeneity of the mixed cohort. As demonstrated in previous studies, systematic reviews and meta-analyses will be essential to provide more accurate estimates of success rates and other outcome measures for MCS in the treatment of trigeminal pain syndromes.
Longevity of pain relief and safety
A common concern regarding invasive MCS is its longevity, as some patients in the literature have only experienced temporary pain relief. Reference Sachs, Babu, Su, Miller and Henderson26,Reference Cioni and Meglio27 This study contributes to the growing body of evidence supporting the effectiveness of MCS as an intervention that provides sustained pain relief. Reference Henssen, Kurt, van Cappellen van Walsum, Arnts, Doorduin and Kozicz28,Reference Aibar-Durán, Villalba Martínez, Freixer-Palau, Araus-Galdós, Morollón Sanchez-Mateos and Belvis Nieto29 Pain relief was maintained in all patients during the median 22-month follow-up period (range: 12–40 months) and extended up to 40 months with progressive improvement in the case of Patient 1. Previous research has proposed that a tolerance phenomenon could explain the diminished effect of MCS over time. Reference Sachs, Babu, Su, Miller and Henderson26,Reference Aibar-Durán, Villalba Martínez, Freixer-Palau, Araus-Galdós, Morollón Sanchez-Mateos and Belvis Nieto29 Conversely, in this study, the pain relief was maintained during the 12-month follow-up period, with three of five patients’ VAS scores improving through the study period from 6 to 12 months. We propose that continued adjustments and reprogramming of their devices, in addition to an increase in neuronal plasticity and synaptic remodeling in critical pain modulation regions could explain this phenomenon. Reference Cha, Um, Kwon, Nam and Lee30 Recently, Aibar-Duráne et al. demonstrated that 68.4% (n = 13/19) of patients with PTN were responders to MCS at the 6-month postoperative mark, defined as a pain improvement greater than 50% on a numerical rating scale. Similarly, most of these responders (58%, n = 11/19) sustained their positive response at the last follow-up, with a mean duration of 51 ± 23 months. Reference Aibar-Durán, Villalba Martínez, Freixer-Palau, Araus-Galdós, Morollón Sanchez-Mateos and Belvis Nieto29 Complications of invasive MCS arise in approximately 15% of patients, commonly including temporary, focal seizures and wound infection. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 None of our patients had immediate or delayed complications from the procedure.
Opioid use reduction
The prolonged use of opioids for chronic, non-malignant pain is associated with suboptimal pain management, self-rated health deterioration and an overall decline in quality of life. Reference Eriksen, Sjøgren, Bruera, Ekholm and Rasmussen31 In many cases, long-term narcotic therapy falls short of achieving essential treatment goals, such as pain relief, enhanced functional capacity and improved quality of life. Reference Eriksen, Sjøgren, Bruera, Ekholm and Rasmussen31,Reference Veiga, Monteiro-Soares, Mendonça, Sampaio, Castro-Lopes and Azevedo32 As a measured outcome in various trials assessing intervention effectiveness in reducing opioid consumption, the reduction in MED (mg/day) serves as a valuable indicator. Reference Eccleston, Fisher, Thomas, Hearn, Derry and Stannard23 In this study, all patients (n = 5) achieved the secondary outcome of a 60% reduction in opioid consumption in MED per day. Notably, the average reduction in opioid use was 79 ± 62 mg/day, corresponding to a 93 ± 13% reduction in narcotic use following 12 months of MCS. We contend that the reduction in opioid use (mg/day) stands as a significant marker of therapeutic benefit, given the association between high doses and prolonged use with opioid-related adverse effects and harms, including dependency and overdose. Reference Chou, Turner, Devine, Hansen, Sullivan and Blazina33
MCS: a Canadian perspective
MCS has previously been utilized and described in Canada, despite its current off-label use. Slotty and colleagues in 2015 described seven patients who were successfully implanted with MCS, including five cases of atypical facial pain, while also describing the effect of stimulation parameter modifications on pain control. Reference Slotty, Chang and Honey34 In constrast, Radic and colleagues proposed that MCS was inefficient for upper limb pain after failing to demonstrate a benefit in six Canadian patients. Reference Radic, Beauprie, Chiasson, Kiss and Brownstone35 The results demonstrated here, as well as those synthesized from the literature, Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4,Reference Mo, Hu, Zhang, Wang, Liu and Zhao5 suggest that MCS could offer significant benefit to patients suffering from trigeminal neuropathic pain of various etiologies. Nonetheless, more studies from within Canada are necessary to increase awareness of its potential benefits and further our understanding of its effectiveness and risks. Building on Canada’s legacy in functional neurosurgery – ranging from Wilder Penfield’s groundbreaking advancements in epilepsy surgery to Claude Bertrand’s contributions to stereotactic and movement disorder surgery Reference Parrent, Lozano, Gildenberg and Tasker36 – we believe Canada is uniquely positioned to make meaningful contributions to the existing literature. Furthermore, we hope this work will inspire other Canadian research groups to investigate the potential of MCS in addressing treatment-refractory cases of PTN.
Limitations and future directions
While our case series demonstrated promising outcomes for MCS in the management of PTN, its small sample size (n = 5) limits the conclusions that can be drawn from it. Additionally, the cohort included patients with complex medical histories, encompassing PTN of various etiologies (e.g., prior MVD, dental avulsion, herpes zoster), which makes it challenging to draw conclusions from the results in aggregate. The lack of a control group and randomization, inherent to retrospective case series, further reduces the strength of the scientific evidence presented. Such limitations, including small and heterogeneous sample sizes, are consistent with the challenges observed in most studies within the current body of literature. Reference Henssen, Kurt, van Walsum, Kozicz, van Dongen and Bartels4 While larger trials would be ideal to advance our understanding of the differential effects of MCS on PTN of varying etiology, meta-analyses that synthesize data from numerous smaller studies such as ours have shed light on the efficacy of MCS in aggregate and may continue to do so for the time being. The duration of MCS analgesia is still unclear, and the limited follow-up time in our study (12–40 months) restricts us from commenting on effects beyond this time frame. We plan to continue monitoring the cases presented here, as well as any new PTN patients treated with MCS, to assess long-term efficacy. We believe this ongoing follow-up is crucial for other research groups, enabling the pooling of long-term results through meta-analysis. Once larger studies are feasible, randomized trials evaluating different stimulation protocols would represent a promising direction for future research.
Conclusion
MCS emerges as a promising therapy in cases of refractory PTN. This case series from Atlantic Canada adds to the growing body of evidence supporting the long-term efficacy of transcranial cortical motor stimulation and suggests that patient outcomes may improve over time. This research underscores the need for further clinical trials, ideally multicenter studies in Canada, to thoroughly evaluate the efficacy and safety of this intervention.
Author contributions
JS: Study design, literature review, data collection and analysis, manuscript redaction and review.
LB: Literature review, data analysis, revised manuscript redaction.
SE: Literature review, original manuscript redaction.
AEH: Study design, patient recruitment, data collection, manuscript review.
Funding statement
The funds for the device implementation were derived from the yearly neuromodulation budget of the Moncton City Hospital.
Competing interests
AHE has received research funding from Abbott within the past 36 months; however, this funding is unrelated to the present study. The authors affirm that the research was conducted without any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Ethics statement
The patients/participants provided their written informed consent to participate in this study.








