Idiopathic intracranial hypertension (IIH) is defined as elevated intracranial pressure (ICP) in the absence of an identifiable cause. Reference Thurtell1 An aggressive or “fulminant” course has been described and requires prompt recognition to initiate treatment to preserve vision. Reference Thambisetty, Lavin, Newman and Biousse2 The goal of this study was to report on consecutive cases of IIH with a fulminant course (less than 4 weeks between the onset of initial symptoms and severe visual symptoms) and new onset severe anemia (hemoglobin below 80 g/L) at presentation to lend additional evidence to this topic.
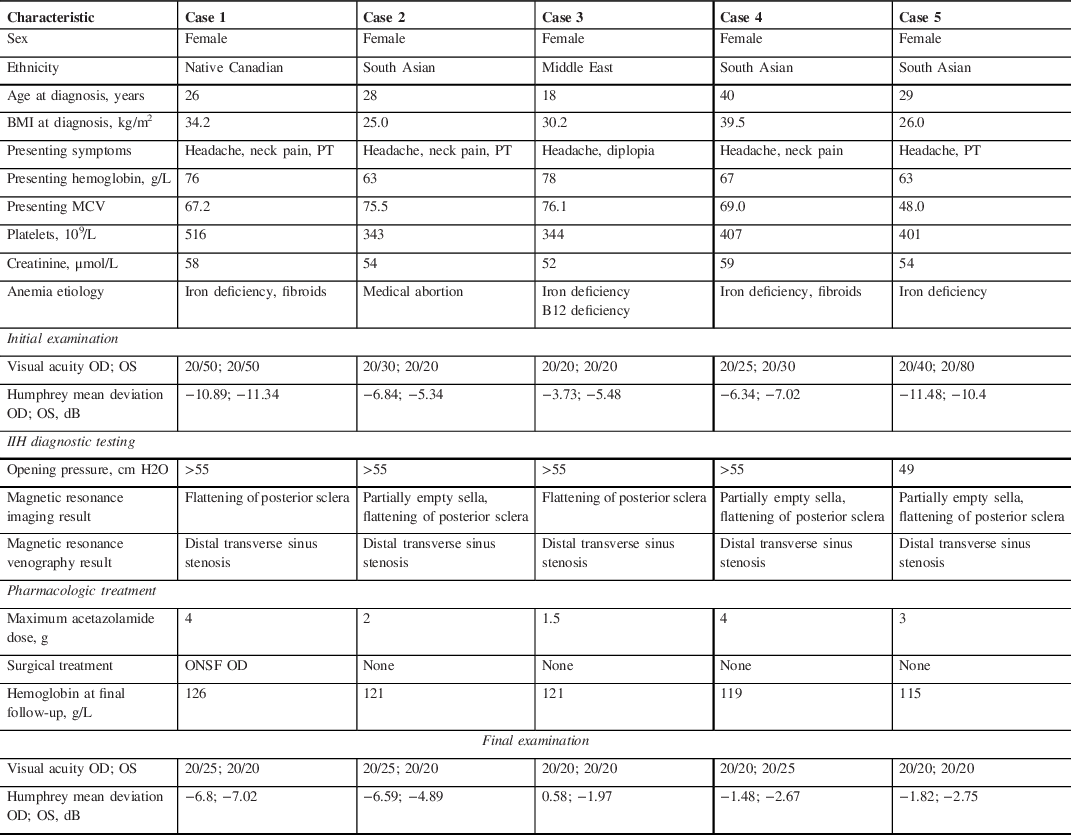
A total of five female patients were included in this report (Table 1). Average age was 28.2 years (range 18–40), mean body mass index (BMI) was 31.0 kg/m2 (range 25–39.5) and mean duration of symptom onset was 2.5 weeks (range 1–4). Renal function was normal in all patients and no patients had known obstructive sleep apnea.
Table 1: Details of five patients with anemia and fulminant idiopathic intracranial hypertension
BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cmH2O, centimeters of water; dB, decibels.
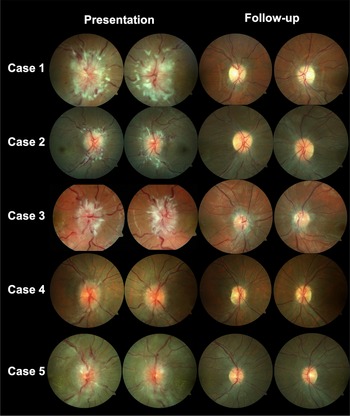
A 26-year-old woman presented with new onset headaches, neck pain, and pulsatile tinnitus (Case 1). She was found to have a hemoglobin of 76 g/L (MCV 67.2), which was unknown to her and related to new menorrhagia from fibroids. After initial treatment for presumed otitis media, she returned with blurred vision and was found to have bilateral optic disc edema (ODE) and was diagnosed with IIH. She was treated with IV iron, acetazolamide and underwent a right optic nerve sheath fenestration. The optic disc edema and symptoms resolved after 1 month with normalization of her hemoglobin (Figure 1).

Figure 1: Fundus photography for cases 1–5 demonstrating bilateral optic disc edema.
A 28-year-old woman underwent an elective medical abortion with Misoprostol treatment (Case 2). Her hemoglobin was 128 g/L prior to treatment. After initiating this medication, she had continuous menstrual bleeding for 2.5 months and underwent dilation and curettage two weeks prior to presentation. One week later, she developed new onset headaches, neck pain, blurred vision and pulsatile tinnitus. She was found to have bilateral ODE and diagnosed with IIH. Her hemoglobin was found to be 63 g/L (MCV 75.5) and was treated with IV iron and acetazolamide was initiated. Her symptoms, papilledema and anemia resolved after 1 month.
An 18-year-old woman with known anemia (iron deficiency related to menorrhagia and vitamin B12 deficiency) developed worsening headaches 2 weeks prior to presentation (Case 3). She had not followed up regularly with her physician regarding her anemia treatment. One week prior to presentation, she developed horizontal binocular diplopia and blurred vision. She was found to have had a limitation of abduction in the right eye and bilateral ODE and diagnosed with IIH. Her hemoglobin at that time was 76.1 g/L (MCV 76.1), which was lower than her previous hemoglobin of 102 g/L at 2 months prior. She was treated with IV iron, IM vitamin B12, and acetazolamide. Her symptoms, papilledema and anemia resolved after 6 weeks.
A 40-year-old woman developed menorrhagia related to fibroids (Case 4). She underwent a uterine biopsy and her hemoglobin was found to be 53 g/L (MCV 69) at that time. She was started on tranexamic acid and was given 1 unit of packed red blood cells (PRBCs) and a combined oral contraceptive pill. Three weeks after her uterine biopsy, she developed new onset headaches, neck pain, and blurred vision. She later returned to the emergency room as she felt lightheaded and was given another 2 units of PRBCs, IV iron, and tranexamic acid. She was found to have bilateral ODE and was diagnosed with IIH. She continued treatment with IV iron and acetazolamide and her symptoms, papilledema and anemia resolved after 2 months. One month later, she underwent a total hysterectomy as a definitive treatment for fibroids.
A 29-year-old woman developed new onset headaches 1 week prior to the presentation (Case 5). She noticed blurred vision for several days and was found to have a bilateral ODE related to IIH and a hemoglobin of 63 g/L (MCV 48), which was lower than previous 6 months prior (114 g/L). She was treated with IV iron and underwent a second therapeutic LP 2 weeks later as she continued on acetazolamide. Her papilledema, symptoms and anemia resolved after 6 weeks. She underwent esophagogastroduodenoscopy and colonoscopy to investigate the source of her anemia and was found to have nonspecific edematous inflammatory changes in the stomach.
We present five patients with a fulminant IIH disease course associated with severe new onset anemia. Two patients were not obese and did not have recent weight gain, suggesting that the anemia was influential in the development of IIH. All patients had resolution of papilledema within 3 months of initiating treatment for anemia, and their hemoglobin increased to normal levels. No patient had significant weight loss, and the time to resolution was much quicker than typically seen, indicating that anemia likely played a role in the pathophysiology of IIH in our cases.
The relationship between anemia and IIH remains controversial. Reference Mollan, Ball and Sinclair3 Our group performed a systematic review of the literature and identified 74 cases of IIH reported in the literature that were associated with anemia, with only 16 of these patients reported to be obese. Reference Yu, Waisberg, Kwok and Micieli4 The most common causes of anemia were iron deficiency. In 60% of cases, anemia treatment without ICP-lowering therapy was initiated and patients demonstrated improvement or resolution of symptoms. Moreover, 15 patients only treated for anemia had repeated LP after treatment and there was a reduction of ICP by 13.5 cmH2O at a mean follow-up of 2.5 months. This evidence supports a direct role of anemia in the development of IIH in some cases.
Four studies compared the prevalence of anemia in IIH patients as compared to a control group; there was a higher prevalence of anemia in IIH patients (20.1%) compared to controls (13.7%). Lin et al. stated that severe anemia may potentially cause IIH hemoglobin values to be extremely low (<8 to 10 g/dL). Reference Lin, Berry, Nakawah, Sadaka and Lee5 The mechanisms underlying the association between anemia and increased ICP remain unknown. It may be related to local hypoxia and increased capillary permeability in the brain or the hypercoaguable state induced by anemia.
Our case series highlights five uncommon presentations of fulminant IIH with new onset severe anemia. This suggests it is important to obtain a CBC in patients with papilledema as the relationship may be causal.
Conflicts of Interest
None.
Statement of Authorship
Conception and design: EW and JAM. Data collection: JAM and IS. Drafting of manuscript: EW, CWY and JAM. Critical revision: JAM, EW, CWY and IS. Final approval: JAM.