Introduction
Following successful clinical trials of monoclonal antibody therapies such as aducanumab, Reference Budd Haeberlein, Aisen and Barkhof1 lecanemab Reference van Dyck, Swanson and Aisen2 and donanemab, Reference Sims, Zimmer and Evans3 disease-modifying therapies (DMTs) for Alzheimer’s disease (AD) have now received regulatory approval in many countries. These therapies, which bind with high affinity to amyloid beta (Aβ) protofibrils or plaques, have been tested in multicenter, randomized, double-blind, placebo-controlled, phase 3 trials that enrolled participants with early symptomatic AD (i.e., mild cognitive impairment/mild dementia). They have all shown, to varying degrees, (a) significant removal of Aβ plaques, as evidenced primarily using positron emission tomography (PET) Aβ imaging, accompanied by (b) significantly slowed clinical progression. Reference van Dyck, Swanson and Aisen2,Reference Mintun, Lo and Duggan Evans4,Reference Sevigny, Chiao and Bussiere5
Participants in these trials had spontaneous or treatment-related adverse events, with some detectable as magnetic resonance (MR) imaging signal abnormalities. These are now referred to as amyloid-related imaging abnormalities (ARIA) and are classified into two types: amyloid-related imaging abnormalities-edema/effusion (ARIA-E) and amyloid-related imaging abnormalities-hemosiderosis/microhemorrhages (ARIA-H). Reference Sperling, Jack and Black6,Reference Filippi, Cecchetti, Spinelli, Vezzulli, Falini and Agosta7 Both forms of ARIA may occur in the same individual. While most ARIA cases in the trials were asymptomatic, symptomatic ARIA-E cases occurred at higher doses, with most, but not all, resolving within 3–4 months or upon treatment cessation. Reference Hampel, Elhage, Cho, Apostolova, Nicoll and Atri8 The presence of prior microhemorrhages on baseline MRI, apolipoprotein E polymorphism and treatment dosage are major risk factors for both ARIA-E and ARIA-H, as well as their severity. Reference Hampel, Elhage, Cho, Apostolova, Nicoll and Atri8 The value of clinical imaging – PET and MR – is therefore twofold: to determine if patients are suitable for treatment initiation and to assess whether they can continue receiving treatment in the face of ARIA risk. Hence, the use of DMTs in AD will require baseline pretreatment and follow-up MRI during treatment, as well as some form of Aβ evaluation, preferably using PET. Reference Cummings, Apostolova and Rabinovici9,Reference Cummings and Salloway10
In the Canadian context, both recommendations would place a significant burden on radiological and nuclear medicine resources. The availability of MR and PET imaging in Canada varies greatly between provinces, and it is readily recognized that the number of scans required to properly qualify and monitor treatment in DMT candidates adds to the already substantial burden on the capacity of our imaging facilities. Unquestionably, additional research and planning are needed to clarify MRI and PET capacity, with respect to the number of DMT candidates in any given region. Until this research is done, we are unable to comment on the impact of AD DMTs on current MRI and PET wait-list times. Rather, the scientific community can provide partial answers for such clinical questions as imaging protocols, imaging frequency, scanner strength and other parameters that directly impact the quantity, quality and type of imaging that will be required of the clinical imaging ecosystem.
At the Canadian level, the Canadian Consortium for Neurodegeneration in Aging (CCNA) is one of the major networks of academic and clinical researchers devoted to aging and dementia. The breadth and depth of expertise among CCNA members can be harnessed to formulate recommendations based on the most current evidence. Such recommendations could then inform regulatory and other governmental bodies. A recent example in this context was the CCNA’s position statement against Health Canada regulatory approval for aducanumab, based on a lack of evidence to conclude that this DMT met the accepted criteria for clinical efficacy, safety and risks/benefits of therapy for AD. Reference Chertkow, Rockwood and Hogan11 While aducanumab ended up not being approved in Canada (and has now been removed from the American market for nonclinical reasons), a regulatory answer is expected for the two most recent DMTs (lecanemab, donanemab) in Canada, following their approval in the USA. The CCNA therefore identified a need to provide considerations regarding their clinical implementation, as well as suggestions for a Canadian research agenda. Consequently, alongside several other workgroups addressing other aspects of AD DMTs, the CCNA has convened a work group to provide an overview of clinical and scientific challenges related to medical imaging given the potential arrival of new DMTs for AD in Canada.
Methods
Workgroup activities were centered around a Delphi process, as illustrated in Figure 1. The workgroup was created once the CCNA mandate was received on July 24, 2023. Following this call to action, an initial cadre of specialists was recruited from across Canada to represent a range of expertise: radiology, neurology, biomedical engineering, nuclear medicine, MR imaging, and medical physics (cf. Figure 1).
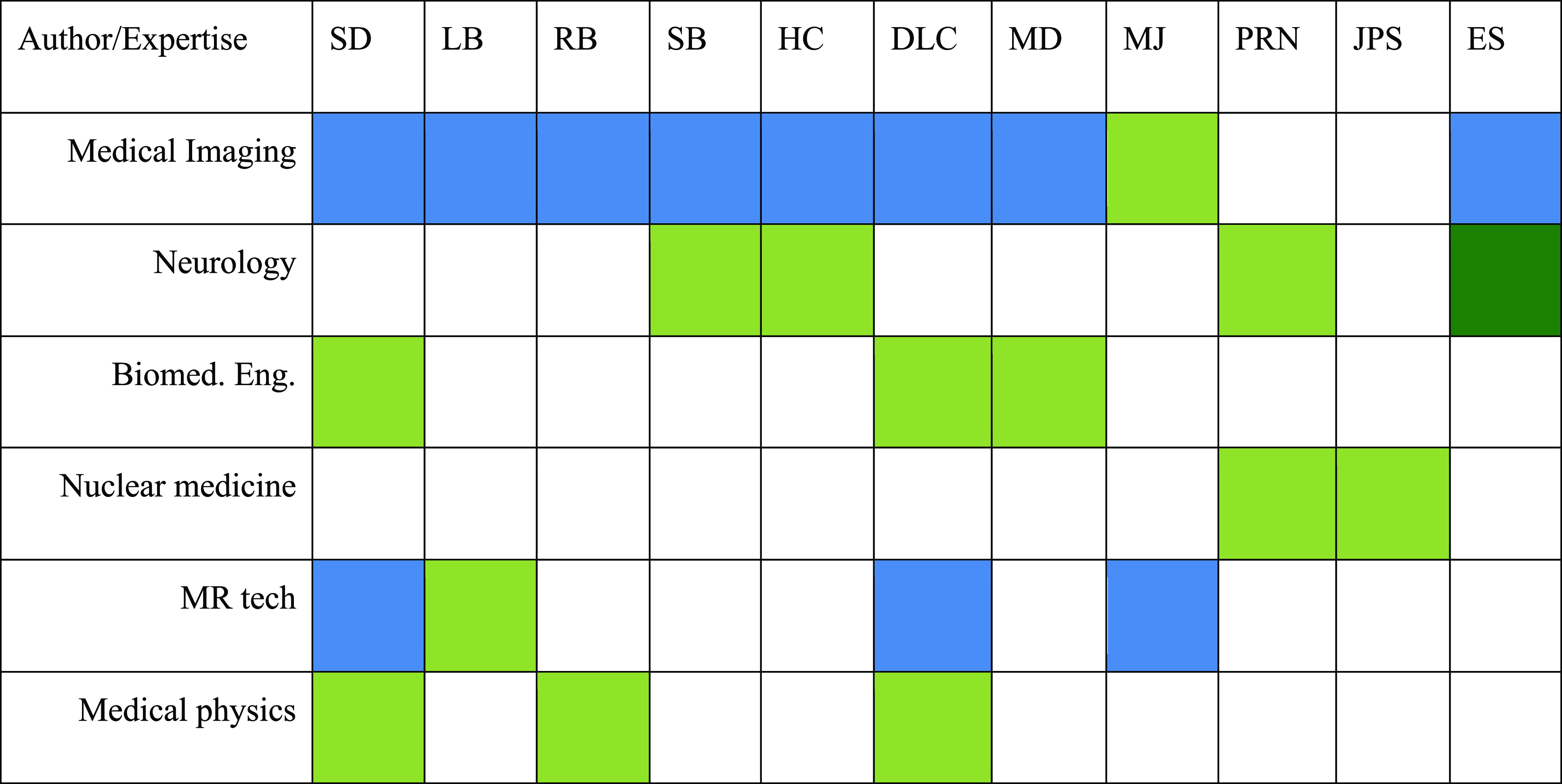
Figure 1. Workgroup members expertise. Green = primary expertise; blue = secondary expertise.
At the starting round, the CCNA mandate as provided was approved by all members unanimously. It was further decided to add MR imaging (e.g., MR technologist) and nuclear medicine expertise to the workgroup. Given that the workgroup was not focused on implementation issues related to access, expertise in epidemiology, hospital administration and health economics was not incorporated.
Following this initial meeting, several issues were raised and formed the core of the Delphi process, with surveys being sent to workgroup members using the SurveyMonkey platform (https://www.surveymonkey.com); answers were collected and analyzed, and a debrief meeting was conducted after each survey to identify questions that remained unanswered or contentious.
Four meetings were held between September 21, 2023 and January 25, 2024. The draft version of this manuscript was edited and circulated to workgroup members in late February to early March 2024 and discussed in Montreal, QC, on March 21, 2024. The final version contains the recommendations from the workgroup, alongside the proportion of experts who agreed with each recommendation. Strong agreement was defined as over 80% agreement among experts, and moderate agreement was defined as 60–79% agreement.
Results
Protocols summary
Tables 1 and 3, respectively, detail salient features of the recent trials of DMTs for AD as well as the accompanying imaging protocols. In all trials, several follow-up MRIs were obtained to screen for the presence of ARIA-E and ARIA-H; however, the exact frequency and timing varied. In fact, it was found that MRI parameters were not specified in sufficient detail in trial publications and supplemental trial protocol documents to reproduce the drug-specific MRI protocol in routine practice; for example, there were incomplete details on the MRI field strength, slice thickness and sequence types. Notwithstanding, it appears likely that clinical trial design was influenced by the Sperling 2011 consensus recommendations for standards for MRI screening for ARIA (required minimum field strength of 1.5T, maximum slice thickness of 5 mm (without any specification on slice gaps) and GRE “recommended” as it was “presently available on any scanner worldwide”). Reference Sperling, Jack and Black6
Table 1. DMT trials summary
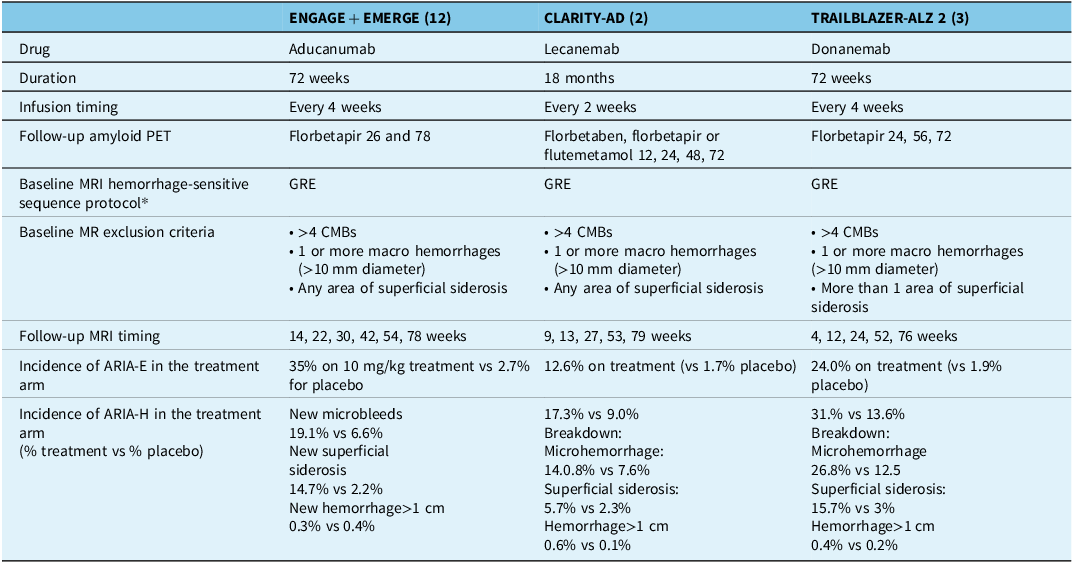
DMT = disease-modifying therapy; PET = positron emission tomography; CMB = cerebral microbleed; ARIA-E = amyloid-related imaging abnormalities-edema; ARIA-H = amyloid-related imaging abnormalities-hemorrhagic; GRE = MRI T2*-weighted gradient recalled echo.
Recommendation 1: Trials of AD DMTs should report complete MRI sequence parameters, in either the main trial publication or supplemental documents and in sufficient detail to allow their reproduction in clinical practice.
Tailoring monitoring protocols by drug
Apart from the specifics of the images to be acquired, the issue of tailored monitoring was quickly raised by the expert panel. Effectively, each DMT trial used a slightly different follow-up imaging protocol that depended in part on their expected risk and efficacy profiles. Should this approach be maintained as these drugs are released to the general patient population?
On the one hand, recommendations should be based on the evidence collected in the trials, and hence, the use of each drug should incorporate the same monitoring protocol as trialed. Each drug has – and future drugs will also exhibit – different safety and efficacy profiles, and these drive the frequency, comprehensiveness and evaluation of monitoring to be performed. Not all risk profiles were “discovered” in the trials, as the cohorts were well characterized and, by design, as homogeneous as possible. Prudence therefore suggests that we do not venture away from what, at a minimum, was used for the trialed group. On the other hand, this approach will rapidly complicate an already complex provisioning system for imaging services. A standardized protocol, for all DMTs, would be more practical and clinically easier to deliver and allow for head-to-head comparisons of biomarkers of interest.
Recommendation 2: Tailored monitoring protocols should be used for each drug that follows regulatory guidelines if issued or appropriate use recommendations if regulatory guidelines are not available. A common protocol may be considered when more information becomes available on drug safety, efficacy, side effects and risk profiles (91% agreement).
Tailoring monitoring protocol by risk profiles
The risk profiles of individuals undergoing treatment can vary significantly based on factors such as APOE status, sex, ethnicity and preexisting cerebrovascular conditions. These risk factors can influence both the safety and efficacy of the treatment, making it crucial to consider them when designing monitoring protocols. Additionally, most ARIA emerges in the first months of treatment, raising the question of whether longer-term routine surveillance is always necessary and whether it is cost effective. Reference Salloway, Chalkias and Barkhof12
Currently, the available data on how these risk factors specifically impact the safety and efficacy of DMTs are limited. As a result, the expert panel concluded that there is insufficient information to justify deviating from the established monitoring protocols at this time. However, the importance of continuing to explore adverse events in immunotherapy trials to better understand risks and inform future treatments is recognized, alongside further studies to better understand how these risk factors interact with DMTs.
By maintaining current protocols until more data are available, we ensure patient safety and the integrity of the monitoring process. Future research will provide the necessary insights to tailor monitoring protocols more precisely to individual risk profiles, enhancing the overall effectiveness and safety of DMTs for AD.
Recommendation 3: Further studies of the safety, efficacy, side effects and risk profiles associated with various risk factors should be performed before deviating from the current monitoring protocols (100% agreement).
Tailoring monitoring protocols by treatment efficacy
The efficacy of DMTs for AD can vary, which raises the question of whether monitoring protocols should be adjusted based on the observed efficacy of each treatment. For instance, a reduction in the frequency of scans might be considered if treatment is shown to be less effective as it is liable to be discontinued. However, this approach must be carefully evaluated to ensure patient safety and treatment efficacy.
The expert panel discussed whether individualized or group-level adjustments to monitoring protocols based on treatment efficacy are warranted. Each DMT exhibits different safety and efficacy profiles, influencing the frequency and comprehensiveness of the required monitoring. The consensus was that the monitoring protocol should remain consistent regardless of the perceived efficacy of an individual’s treatment. This ensures that any adverse effects or complications are promptly detected and managed, maintaining the overall safety and well-being of patients. Further, maintaining a consistent monitoring protocol allows for standardized data collection and comparison across different treatments, facilitating a more accurate assessment of long-term safety and efficacy. It also ensures that all patients receive the same level of care and monitoring, regardless of the specific DMT they are receiving.
Recommendation 4: The monitoring protocol should not be changed even if treatment with any DMT is not shown to be optimally effective (100% agreement).
Scanner magnetic field strength
Clinical MR scanner magnetic field strengths, expressed in tesla (T), range from low-field systems (0.0625T–1.0T) to higher-field systems (3.0T). A survey of 455 Canadian medical facilities (e.g., hospitals, clinics) with MRI units found that most scanners (80.9%) operated at 1.5T field strength, with 17.1% of centers housing a 3T system. 13 Few centers operated at or below 1T (0.9%). It was recognized that 3T scanners provide a higher contrast-to-noise ratio, which can improve the detection rate and visibility of lesions – for example, cerebral microbleeds. Reference Cummings and Salloway10 However, there are more artifacts at higher field strength, Reference Westbrook, Roth and Talbot14 while some implants/devices only have conditional approval at lower field strengths.
Recommendation 5: MRI screening and monitoring can be performed on either 1.5T or 3T scanners, provided that protocols are adapted to acquire similar tissue contrasts at comparable resolution (100% agreement).
MR protocol management and general definition
Standardizing MR protocols is essential to simplify clinical implementation, enhance reproducibility across different centers and facilitate the training of radiologists. The adoption of common standards ensures that imaging data are consistent, reliable and comparable, which is critical for monitoring the effects of DMTs. The MR protocol should conform to published imaging standards, such as the STRIVE/STRIVE-2 criteria for small vessel disease. Reference Duering, Biessels and Brodtmann15,Reference Wardlaw, Smith and Biessels16 Standardization includes the use of specific sequences (see below) that are necessary for accurate diagnosis and monitoring of ARIA and other biomarkers. Reference Roytman, Mashriqi and Al-Tawil17 By adhering to standardized protocols, we can improve the quality and consistency of imaging data, creating the conditions to improve detection and monitor changes over time, ensuring that patients receive the best possible care.
Recommendation 6: Protocols should be standardized across platforms, scanner strength and DMTs (100% agreement).
Recommendation 7: Patients should be scanned at screening and then at follow-up/ARIA visits on the same scanner and with the same imaging protocol to ensure consistency (100% agreement).
Optimal protocol length
The length of an MR protocol is a critical factor in clinical feasibility and patient compliance. It is essential to balance the need for comprehensive data collection with the practical constraints of clinical settings and patient comfort. Modern MR scanners, equipped with advanced software and hardware, allow for efficient data acquisition within shorter timeframes while maintaining high image quality and resolution.
The expert panel agreed that an MRI protocol lasting between 20 and 30 minutes is both clinically feasible and sufficient to collect all relevant information necessary for monitoring DMT delivery. This duration is manageable for patients and ensures that the imaging process is not unduly burdensome for clinical workflows.
Recommendation 8: Provided MR scanners are maintained to a contemporary standard with respect to software/hardware, a protocol lasting 20–30 minutes is both clinically feasible and sufficient with modern acquisition approaches to collect all relevant information (100% agreement).
Specific MR protocol sequences
Following STRIVE-2, Reference Duering, Biessels and Brodtmann15 an MR protocol should include (1) a 3D T1-weighted (T1w) high-resolution anatomical image, to “discriminate lacunes from perivascular space, to discriminate gray from white matter, to discriminate cortical microinfarct and to measure brain tissue volumes”; (2) a T2-weighted (T2w) acquisition, to “characterize brain structure, to differentiate lacunes from white matter hyperintensity and perivascular space and to identify old (i.e., chronic) infarcts”; (3) a fluid-attenuated inversion recovery (FLAIR) image, to “identify white matter hyperintensity, established cortical or large subcortical infarcts and cortical microinfarct and to differentiate white matter hyperintensity from perivascular space and lacunes”; and (4) a diffusion-weighted imaging (DWI) acquisition, to “detect acute ischemic lesions, positive for up to several weeks after cerebrovascular event.” These sequences were considered necessary by all experts.
It was mentioned that 3D FLAIR was now becoming more prevalent in clinical practice but was not judged essential in the DMT context. 3D isotropic acquisitions in general are more flexible and reproducible longitudinally as the images can be reformatted in any direction, including to match previous positioning. Alignment (at console) with baseline images is recommended. The most subtle cases of ARIA-E can involve an effusion in one or two sulci or loss of the sulci without parenchymal signal hyperintensity from very early edema. Reference Barakos, Sperling and Salloway18 The superior contrast resolution of 3D FLAIR Reference Chagla, Busse, Sydnor, Rowley and Turski19 would demonstrate those changes better but could also introduce more false positives. Subtle ARIA is not that common. Most stroke imaging protocols that sites would use to screen ARIA already incorporate 2D FLAIR routinely.
To detect intracerebral hemorrhage, cerebral microbleed and cortical superficial siderosis – ARIA-H – two options are available. T2* gradient recalled echo (GRE) was the standard used when the consensus paper on ARIA was published in 2011, Reference Sperling, Jack and Black6 as GRE was what most centers used at the time, and hence, all clinical trial protocols used GRE (cf. Table 2). On the other hand, new methods such as susceptibility-weighted imaging (SWI) are more sensitive Reference Puy, Pasi and Rodrigues20 and are now widespread in routine practice. The prevalence and number of detected microbleeds can vary by twofold or more across sequence types. Reference Sepehry, Lang, Hsiung and Rauscher21 However, SWI suffers from drawbacks, such as the difficulty of distinguishing between cross sections of venules and microbleeds. Reference Shams, Martola and Cavallin22 There is also insufficient information on the effects of slice thickness and field strength on the sensitivity and specificity of ARIA-H detection by GRE and SWI.
Table 2. Imaging protocols
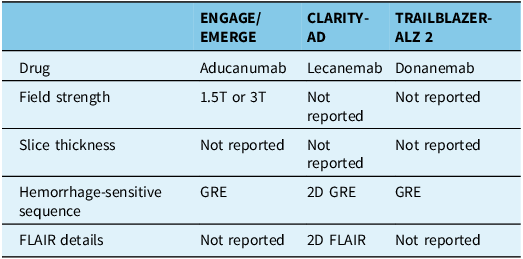
GRE = MRI T2*-weighted gradient recalled echo; FLAIR = MRI fluid-attenuated inversion recovery; T = tesla.
Recommendation 9: The following acquisitions should be included in a base MRI protocol: 3D T1-weighted, 2D FLAIR, 2D T2*GRE and DWI (100% agreement).
Recommendation 10: Centers are encouraged to perform a 3D rather than 2D FLAIR, as well as acquire a susceptibility-weighted image over and above a T2* GRE if possible (91% agreement).
Recommendation 11: Further studies on the sensitivity and specificity of high-resolution susceptibility imaging for ARIA-H detection should be performed (100% agreement).
Operational definition of ARIA-E and ARIA-H
Radiological review and reporting will need to be specific enough to match trial-related criteria for eligibility and for ARIA severity. For example, to determine treatment eligibility and to grade the severity of ARIA-H, the exact number of prevalent or new microbleeds is needed; considering this, interpretations such as “there are a few scattered microbleeds” will need to be replaced by precise counts. This presupposes that precise definitions are available, including the minimum size for a microbleed, as there appears to be no clear consensus on the lowest dimension threshold (e.g., 10 mm diameter cutoffs); clinical reading is further complicated by the presence of “bloom” that can vary with echo time. This lack of clarity will directly impact accessibility to treatment, as the criteria for most AD DMTs require patients to have fewer than four microbleeds. Additionally, the largest dimension of ARIA-E on FLAIR should be measured in cm and reported.
Radiologists who interpret imaging of patients receiving AD DMTs should have sufficient background training and experience in neuroimaging. Certification in neuroradiology (accredited fellowship or residency) and a predominant practice focus in neuroradiology where radiologists are reporting sufficient volumes of neuroimaging are highly recommended. Given that approved therapies will be relatively new to the market in Canada, even experienced neuroradiologists will require additional, specific training through accredited continuous medical education activities regarding the standardized reporting of pretreatment, baseline MRI studies to determine if patients are suitable for therapy, as well as for ongoing monitoring during therapy. They will have to have the necessary knowledge of the spectrum of MRI imaging findings of ARIA (as well as appropriate imaging differentials) and be aware of and utilize standard grading schemes for ARIA-E and ARIA-H in written and/or verbal communication with referring physicians. These requirements may increase the time for radiological review. For centers using electronic health records, the implementation of standardized reporting templates may be useful.
The diagnosis and management of ARIA in asymptomatic and symptomatic patients are heavily dependent on findings obtained using MRI. An integrated, organized, systematic framework for imaging diagnosis, reporting and timely communication between radiologists and referring physicians will facilitate patient care and safety.
Recommendation 12: A consensus conference should be convened on the operational definition of ARIA-H and ARIA-E (91% in agreement).
Recommendation 13: Guidelines should be used to rate ARIA-E and ARIA-H (100% in agreement).
Recommendation 14: Intra- and inter-rater variability in ARIA detection, cross-sectionally and longitudinally, should be studied further (100% in agreement).
Imaging follow-up of ARIA-E and ARIA-H
Monitoring protocols for follow-up of patients with ARIA-H or ARIA-E varied across the different drugs, particularly in the frequency and timing of MRI scans required. Additional follow-up scans were required until the ARIA stabilized (ARIA-H) or resolved (ARIA-E), upon which dosing was resumed. However, more severe ARIA could trigger permanent discontinuation of the drug. Staging symptoms for ARIA (mild, moderate or severe) were also not consistent across trials.
Currently, there are insufficient data to determine whether a single, standard protocol for imaging of ARIA resolution can be used for all drugs. Additionally, the variation in clinical MRI protocols and competency of MRI readers may affect the ability to detect radiological signs of ARIA.
Recommendation 15: Further studies are necessary to provide information for imaging follow-up guidelines of ARIA-E and ARIA-H.
PET imaging
In the anti-amyloid trials, PET was deployed as the main technique for measuring target engagement or efficacy. Treated patients had marked reductions in amyloid signal with most patients achieving essential normalization. In the TRAILBLAZER-ALZ 2 trial, treatment with donanemab was stopped if the amyloid-PET signal was less than 11 centiloids at week 24 or 52 or between 11 and 25 centiloids on both; 29.7% of patients achieved this level of amyloid clearance by 24 weeks and 76.4% by the end of the trial. The committee agreed that this individualized treatment approach, stopping therapy after amyloid is removed, is a promising means to reduce resource use and lower patient burden. Whether patients in whom amyloid is removed require future PET surveillance for re-accumulation, and the optimal frequency and timing of that surveillance, is not currently known.
The availability of PET imaging across Canada is limited to 45 cameras, with 24 in Quebec and 12 in Ontario. 13 Florbetaben is the sole imaging agent for beta-amyloid used clinically, with high sensitivity and specificity exceeding 90%. Reference Sabri, Seibyl, Rowe and Barthel23 The production of florbetaben is confined to Quebec and Ontario. Although cyclotrons are present in other regions (e.g., Vancouver, Edmonton, Winnipeg), enabling potential synthesis at these sites, scanning capacity is restricted. Oncology currently maximizes the use of these resources, and a significant increase in the number of scans would surpass capacity limits. Furthermore, there are personnel shortages in nuclear imaging technologists across all provinces. While physicians could increase local scan reading, training is necessary for readers.
For PET amyloid imaging, the SNMMI Procedure Standard/EANM Practice Guideline for Amyloid PET Imaging of the Brain (version 1.024) should be used as a guide to acquiring/processing/interpreting those studies. Although most of the trials deploy centiloids as an outcome measure, this amyloid-PET metric is not currently attainable in clinical practice. Reference Bollack, Collij and Garcia25,Reference Tudorascu, Minhas and Lao26
Perfusion SPECT cannot be considered as an alternative for amyloid PET. Further, there is no evidence supporting a role for tau or fluorodeoxyglucose PET in indicating or monitoring patients undergoing anti-amyloid therapies, although phase 3 trials for donanemab and lecanemab suggested that tau PET might play a role in patient selection or monitoring disease progression. Reference van Dyck, Swanson and Aisen2,Reference Mintun, Lo and Duggan Evans4,Reference Sevigny, Chiao and Bussiere5
Recommendation 16: Acquisition of an amyloid-PET scan before beginning therapy should be obtained whenever this is practically available, as repetition of this test during therapy would help directly assess the extent of plaque removal, guiding a decision on whether therapy should be continued or discontinued (100% agreement).
Discussion
Summary
The recommendations from the CCNA DMT Imaging Workgroup (Table 3) underscore the critical role of imaging in the context of DMT for AD. They emphasize the need for tailored monitoring protocols that align with the specific risk and efficacy profiles of each DMT, as well as the importance of standardizing MRI acquisition protocols across various platforms and scanner strengths. This approach aims to ensure both the safety of initiating and continuing treatments and the effectiveness of the therapies by monitoring ARIA and the removal of Aβ plaques.
Table 3. Summary of recommendations
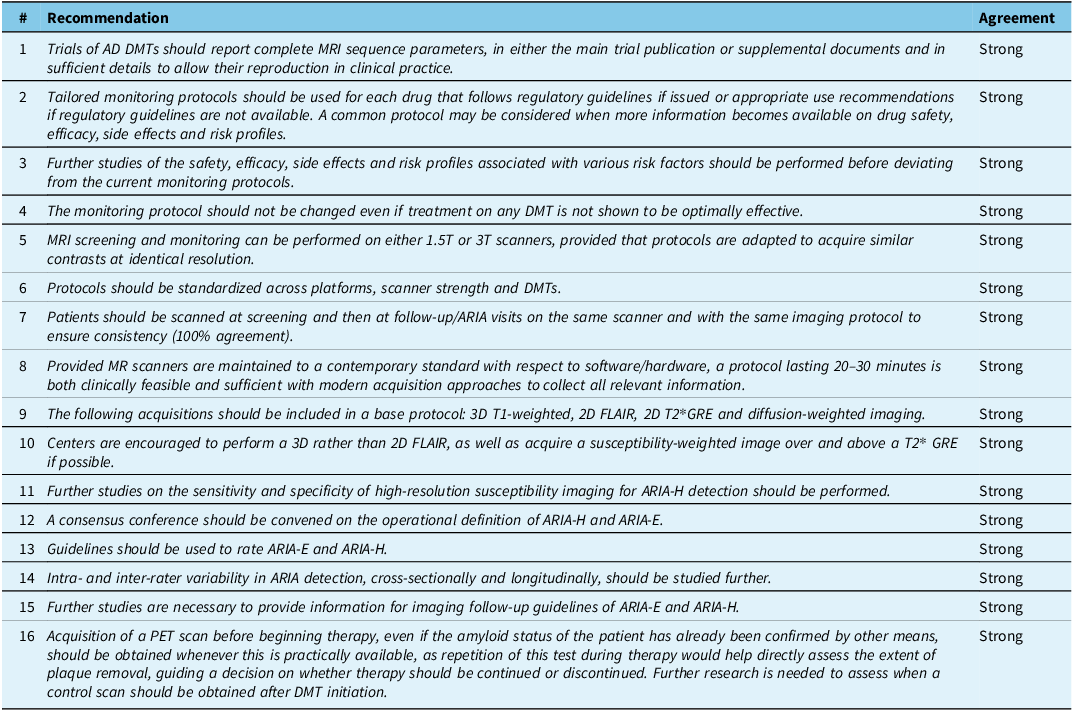
Strong: >80% agreement.
AD = Alzheimer’s disease; ARIA = amyloid-related imaging abnormalities; ARIA-E = amyloid-related imaging abnormalities-edema; ARIA-H = amyloid-related imaging abnormalities-hemorrhagic; DMT = disease-modifying therapies; GRE = MRI T2*-weighted gradient recalled echo; FLAIR = MRI fluid-attenuated inversion recovery; PET = positron emission tomography; T = tesla.
Explanation and comparison of findings
The findings and recommendations of the CCNA Workgroup are consistent with existing literature on the importance of imaging in the monitoring and assessment of DMTs for AD. For instance, they align with previous studies that have shown the significance of detecting ARIA using MRI and the critical role of PET imaging in evaluating the efficacy of amyloid beta removal. By comparing the imaging protocols used in trials for aducanumab, lecanemab and donanemab, the workgroup supports a drug-specific approach to monitoring while also advocating for standardized imaging protocols to facilitate clinical implementation and ensure consistency across different clinical settings.
Future directions
The CCNA Workgroup found many areas for future research (Table 4). This should include a focus on further refining imaging protocols to enhance the detection and management of ARIA, studying the sensitivity and specificity of high-resolution SWI for detecting ARIA-H and developing operational definitions suitable for artificial intelligence applications. The schedule to be followed when using amyloid PET for assessing DMT efficacy also remains to be established. Additionally, more data on the safety, efficacy and side effect profiles associated with various risk factors, such as APOE status and preexisting cerebrovascular conditions, are needed. These efforts will help ensure that imaging protocols remain effective and relevant as new DMTs for AD continue to emerge.
Table 4. Important research questions for future study

AD = Alzheimer’s disease; ARIA = amyloid-related imaging abnormalities; ARIA-E = amyloid-related imaging abnormalities-edema; ARIA-H = amyloid-related imaging abnormalities-hemorrhagic; DMT = disease-modifying therapies; GRE = MRI T2*-weighted gradient recalled echo; FLAIR = MRI fluid-attenuated inversion recovery; PET = positron emission tomography; SWI = MRI susceptibility-weighted imaging.
Study limitations
The recommendations presented are based on current evidence from clinical trials and expert consensus, which introduces certain limitations. The availability of imaging resources varies significantly across Canada, potentially affecting the uniform implementation of these protocols. Moreover, as the long-term safety and efficacy of DMTs are still under investigation, the proposed imaging protocols may need to be adjusted as new data become available. Additionally, the reliance on expert opinion and consensus may introduce biases that could affect the generalizability of these recommendations.
We elected not to discuss the implementation issues posed by the introduction of DMT drugs for AD and how they present significant challenges to the Canadian healthcare system, particularly in testing the principle of universal access. While these advancements promise to enhance patient outcomes, they also highlight the existing disparities in healthcare delivery across the country. Access to care will likely be feasible in many urban centers, yet rural and remote regions may face substantial difficulties. To address these inequities, various strategies must be implemented, including an increased investment in local imaging infrastructure, the implementation of telemedicine services (e.g., teleradiology) and targeted training programs for healthcare providers in underserved areas. Additionally, novel models of care, such as integrated care pathways and collaborative networks, could be developed to ensure timely and equitable access to these therapies. Ultimately, this new era of Alzheimer’s treatment will necessitate a reevaluation and adaptation of current healthcare frameworks to uphold the ethos of universal access and provide comprehensive care to all Canadians.
Further, we acknowledge the ongoing controversy surrounding the cost-effectiveness of anti-Aβ immunotherapies (e.g., the NICE draft guidance of September 2024); however, such an assessment falls beyond the scope of this workgroup’s mandate. Our recommendations are focused on the clinical implementation of imaging protocols to ensure patient safety and treatment efficacy in the context of AD DMTs. We encourage further research and policy discussions to address the broader economic implications of these therapies within healthcare systems.
Conclusion
The recommendations presented by the CCNA Imaging Workgroup highlight the critical role of imaging in the context of DMTs for AD. Through a comprehensive analysis of current evidence and expert consensus, these guidelines aim to ensure the safe and effective implementation of DMTs across Canada. Key recommendations emphasize the need for standardized MRI acquisition protocols, tailored monitoring based on risk profiles and the use of appropriate MR scanner strengths to maximize diagnostic accuracy and treatment monitoring.
Implementing these recommendations will require coordinated efforts among healthcare providers, regulatory bodies and policymakers. The establishment of standardized protocols will enhance the consistency and reliability of imaging data, facilitating better clinical decision-making and patient care. Further research is essential to refine these protocols and to address the evolving challenges associated with new DMTs and their monitoring requirements.
Ultimately, the workgroup’s guidelines represent a step forward in optimizing the use of imaging in AD treatment. By adhering to these recommendations, we can pave the way for more effective use of advanced therapies in the fight against AD.
Author contributions
Conception, design, acquisition, analysis or interpretation of data for the work: All authors.
Drafting the work or revising it critically for important intellectual content: All authors.
Final approval of the version to be published: All authors.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Funding statement
This work was supported by a Canadian Institutes for Health Research grant (#CNA-163902) to the CCNA (principal investigator: H. Chertkow).
Competing interests
SD: Officer and shareholder of True Positive MD. Paid consulting for Eisai and Novo Nordisk. Unpaid consulting for Lilly.
LB: No conflict.
RB: Paid consulting for Merck.
SB: Paid consulting for Biogen, Eisai, Lilly, Novo Nordisk and Roche.
HC: Paid consulting for Biogen, Eisai, Lilly and Roche.
DLC: Officer and shareholder of True Positive MD.
MD: No conflict.
MJ: Paid consulting/honoraria for Clario, Biogen, Eisai and Lilly.
PRN: Clinical trial PI and consulting for Biogen, Eisai, Lilly and Novo Nordisk.
JPS: Has collaborated with Optina Dx, a Montreal-based manufacturer of a retinal scanner with potential applications for AD diagnosis and monitoring of DMT efficacy. Paid consultant for Biogen.
EES: Unpaid consulting for Alnylam, Biogen, Eisai and Lilly.







