Approximately 30% of patients with epilepsy have medically refractory seizures. Refractory epilepsy is defined as the failure to achieve sustained seizure freedom despite an adequate trial of two antiepileptic drugs.Reference Kwan, Arzimanoglou and Berg 1 In this group of patients, epilepsy surgery has the potential to control seizures if an epileptogenic focus can be localized.
Traditionally, localization of the epileptogenic focus depends on seizure semiology, scalp video electroencephalography (vEEG), magnetic resonance imaging (MRI), and neuropsychological assessment. However, these traditional methods have their limitations. vEEG poorly measures epileptiform activity in certain cortical areas, such as the opercular regions, mesial hemispheres, and the insula, among others. MRI may fail to demonstrate a structural abnormality. Seizure semiology may implicate different epileptogenic zones due to overlapping semiology or to propagation phenomena.Reference Tufenkjian and Lüders 2
In the absence of an identifiable epileptogenic focus derived from traditional methods, intracranial EEG (iEEG) remains the gold standard in seizure localization. This technique is invasive, but, most importantly, it requires a strong hypothesis regarding the seizure focus.Reference Spencer 3 Consequently, advanced neuroimaging techniques have been used to guide the placement of intracranial electrodes and complement other traditional methods in localizing epileptogenic foci. One commonly used adjuvant imaging technique is positron emission tomography (PET). We have been using 18F-FDG PET computed tomography (CT) since 2011 as part of our presurgical workup of certain patients being considered for epilepsy surgery. This technique images the topographic distribution of glucose (2-[18F]-fluoro-2-deoxy-D-glucose [FDG]) uptake in the brain and provides a picture of cerebral metabolism.Reference Desai, Bekelis and Thadani 4 PET is typically performed in the interictal state with the goal of detecting focal areas of relative hypometabolism.
The specific roles of this technique have not been standardized, and utilization varies among epilepsy centers. In this paper, we aimed to explore the role of PET CT in presurgical evaluation of patients with refractory epilepsy and to compare the accuracy of PET with standard methods (MRI, vEEG, and semiology) for epileptogenic focus localization.
Methods
The database of the epilepsy program at the London Health Sciences Centre (London, Ontario) between September of 2011 and April of 2016 was reviewed. We identified 62 patients with refractory epilepsy who underwent PET. Patients who underwent surgery were followed up at 3 months and onward. Findings on 1.5T MRI, vEEG, and iEEG were analyzed and compared to PET. The outcomes were documented, including seizure freedom after surgical resection (Engel classification I), improvement in seizure frequency, guidance for further investigations, and the exclusion of patients from further evaluation. Informed consents were obtained.
We excluded patients who did not have a standard presurgical workup, were lost to follow-up, refused surgical intervention, or are currently awaiting PET imaging. Descriptive statistics were applied.
Results
Subject Demographics
We identified 62 patients (33 males and 29 females) with refractory epilepsy who underwent PET. The mean age was 34 years (range=20-68), and the mean onset of epilepsy was 17 years of age (range=1-54). Forty-eight patients had temporal lobe epilepsy (TLE) and 14 had extratemporal lobe epilepsy (eTLE).
MRI Findings
A total of 48 patients had unremarkable MRI imaging, while 14 had abnormal MRI findings: 8 had mesial temporal sclerosis, 2 had cortical dysplasia, 1 had a dysembryoplastic neuroepithelial tumor (DNET), 1 had hippocampal asymmetry, 1 had subcortical tubers, and 1 had a temporal ganglioglioma.
PET Results
Thirty-six patients had concordant (or matching) findings on PET and vEEG, while 26 had discordant findings on PET and vEEG.
Of the 36 patients with vEEG and PET concordance, 17 (13 TLE, 4 eTLE) underwent surgical resection of the epileptogenic focus (47.2%), 7 TLE are awaiting surgical resection, 11 (7 TLE, 4 eTLE) were deemed ineligible for surgical resection due to multiple epileptogenic foci identified (an example is shown in Figure 1), and 1 TLE patient with concordant findings is awaiting further assessment with iEEG.
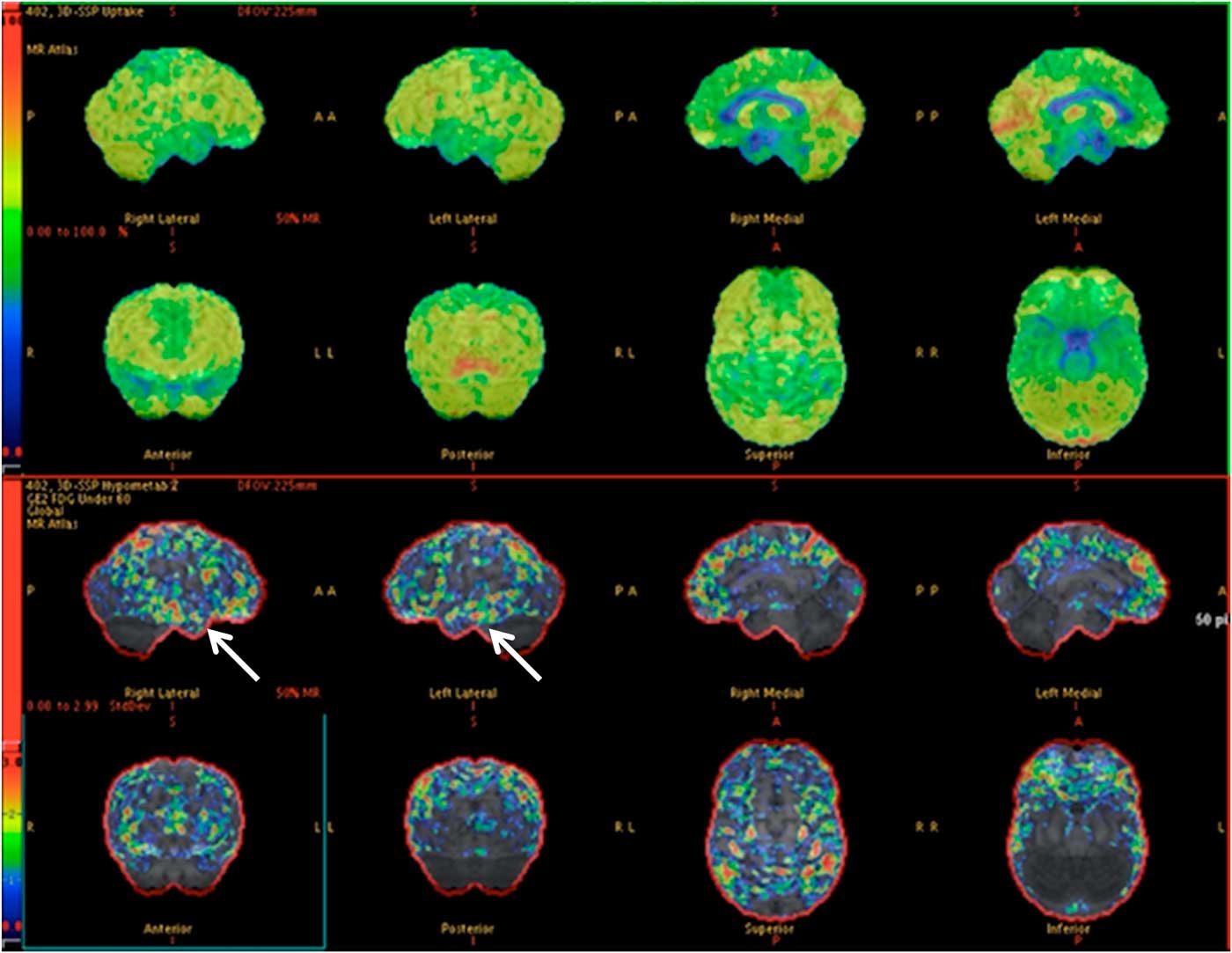
Figure 1 PET images depicting hypometabolism in the mesial aspect of both temporal lobes anteriorly. (Arrows) There is subtle asymmetry, with the right side being slightly more severely affected. The patient (57-year-old male) was deemed ineligible for surgical resection due to bitemporal involvement, which was also found on vEEG (normal MRI).
Of the 26 patients with discordant vEEG and PET, 10 (6 TLE, 4 eTLE) had PET studies reported as normal and 16 (14 TLE, 2 eTLE) as abnormal. In those patients whose PET was reported as normal, 3 became controlled on medications, 2 had multiple epileptogenic foci, 1 had surgical resection of the epileptogenic focus following assessment with iEEG, 3 are awaiting further iEEG monitoring, and 1 declined further investigation. Those 16 patients with an abnormal PET either had more than a single area of hypometabolism or the location of the hypometabolic area differed from the potential epileptogenic foci detected with the use of vEEG, MRI, neuropsychology, and/or SPECT and iEEG.
Surgical Outcomes
Of the 36 patients with concordant PET and vEEG, 17 (13 TLE, 4 eTLE) underwent surgical resection of the epileptogenic focus (Table 1). Six of these patients (5 TLE, 1 eTLE) underwent surgical resection of the epileptogenic focus without iEEG, while 11 (8 TLE and 3 eTLE) underwent the required iEEG.
Table 1 Surgical outcome of patients with concordant PET and vEEG (with and without iEEG)

* A total of 19 patients were excluded: 11 patients with multiple epileptogenic foci identified (nonsurgical candidates), 1 patient awaiting iEEG for further clarification, and 7 patients awaiting surgical resection (did not undergo iEEG assessment).
^ Measured 3 months postoperatively.
Of those who had surgery without iEEG (6/17), 5 became seizure-free (Engel I) and 1 had an improvement in seizure frequency at 3 months. An illustrative case is shown in Figure 2. Of those who required iEEG (11/17), 5 patients became seizure-free (Engel I), while 6 had improvement in seizure frequency at 3 months.
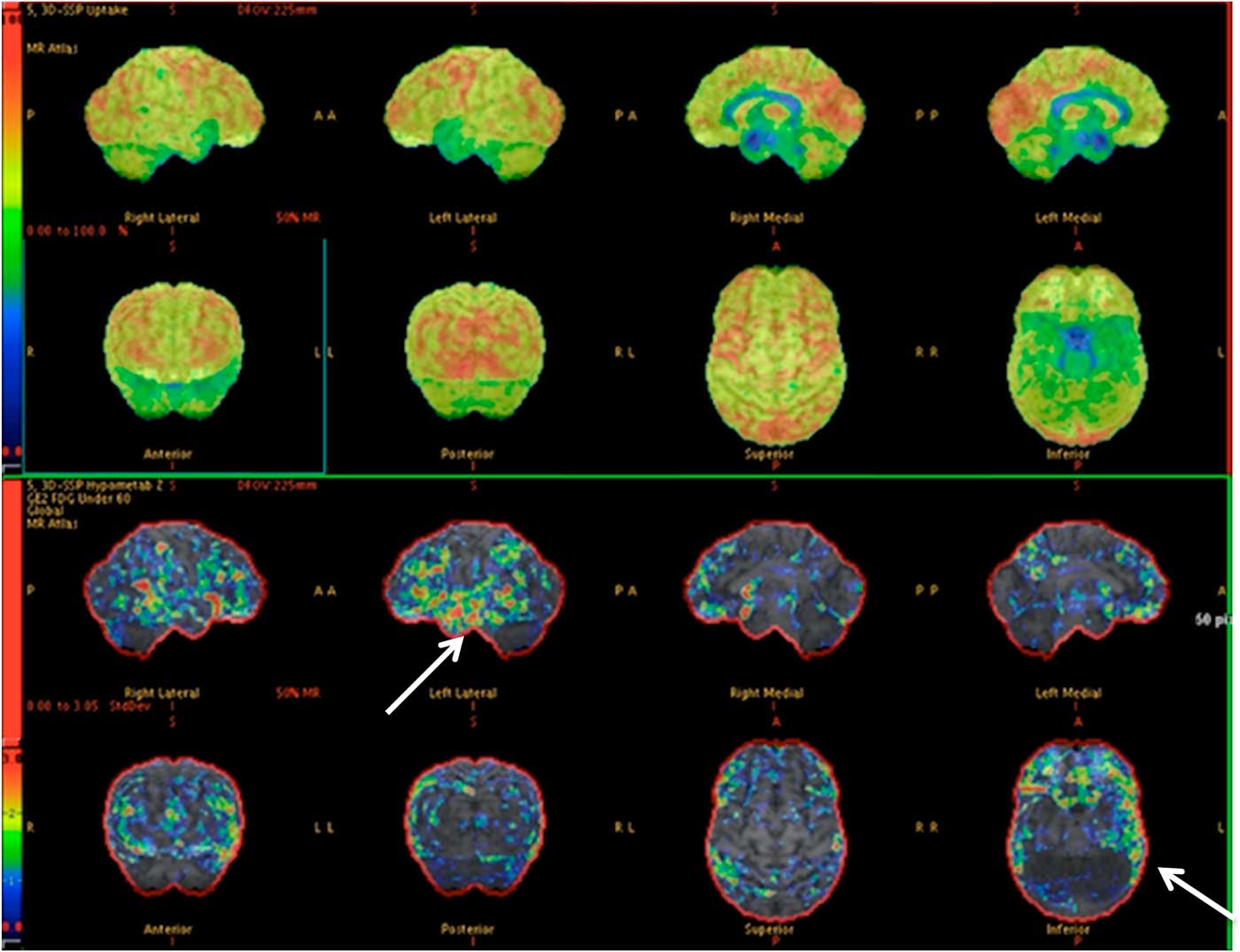
Figure 2 PET images depicting relative left temporal lobe hypometabolism with respect to the right temporal lobe. This was congruent with the vEEG. This patient (35-year-old male) had a left temporal lobectomy and became seizure-free (normal MRI).
Out of the 26 patients with discordant PET and vEEG, 1 had surgical resection of the epileptogenic focus and became seizure-free (with iEEG confirmation) at 3 months, despite having a normal PET. Of the remaining 25 patients, 3 are awaiting further assessment with iEEG, while 22 were not candidates for resective epilepsy surgery.
Discussion
It has been established that MRI evidence of hippocampal atrophy is a strong predictor of excellent postoperative seizure control after an anterior temporal lobectomy.Reference Kuzniecky, Burgard, Faught, Morawetz and Bartolucci 5 However, some individuals with epilepsy do not have an identifiable structural lesion on MRI. In our study population, only 23% of patients had abnormal findings on MRI. It should be pointed out that the MRI scans performed in this series were all with a 1.5T field strength. In one case series, the yield of 1.5T was as low as 17% in detecting lesions in focal epilepsy,Reference King, Newton and Jackson 6 and it is possible that additional lesions might have been identified on 3T MRI that were not seen on the 1.5T scans performed here. It is acknowledged that higher-field MRI, particularly 3T, is preferred for epilepsy imaging and may identify subtle abnormalities such as focal cortical dysplasia.Reference Martinez-Rios, McAndrews, Logan, Krings, Lee and Widjaja 7 It provides higher-resolution imaging and can increase lesion identification and thus improve outcomes following epilepsy surgery.Reference Rubinger, Chan and D’Arco 8 The number of abnormal MRI findings also varies among different care settings due to differences in epilepsy imaging protocols. For example, it has been shown that specific epilepsy-protocol MRI scans identified focal lesions in 85% of patients who previously were reported not to have a lesion with a conventional MRI protocol.Reference Von Oertzen, Urbach and Jungbluth 9
Regardless of MRI type, when traditional methods are incongruent or no structural lesion can be identified, functional imaging such as PET can play a complementary role in localization of the epileptogenic focus and may minimize the need for invasive EEG monitoring or provide guidance for intracranial electrodes placement. It has been previously mentioned in the literature that lateralizing information identified from PET can guide intracranial electrode placement with results comparable to MRI.Reference Knowlton, Elgavish and Bartolucci 10 Unfortunately, very few studies have examined the surgical outcome of patients with normal MRI and matching PET/vEEG findings. In a published review article,Reference Van Paesschen, Dupont, Sunaert, Goffin and Van Laere 13 primary studies compared PET accuracy with surgical outcome. Overall, the percentage of patients in whom PET correctly localized a seizure focus and had a good surgical outcome ranged from 36 to 89%, and it was higher (71-89%) in those with temporal lobe epilepsy.Reference Burneo, Poon, Kellett and Snead 11 Our results demonstrate a favorable surgical outcome in patients with concordant PET and vEEG findings as illustrated in Table 1, indicating that in selected cases iEEG may not be required prior to successful surgical resection. Patients who are awaiting iEEG and anticipating surgical resection were included in the analysis, as the information provided by PET guided the placement of intracranial electrodes. Based on our experience to date, good surgical outcome is anticipated in patients awaiting surgery with concordant PET and vEEG.
The performance of PET imaging in our population would appear to reflect the heterogeneity of epilepsy in our study population, which included a significant number of extratemporal lobe epilepsy patients.Reference Henry, Engel and Mazziotta 12 , Reference Van Paesschen, Dupont, Sunaert, Goffin and Van Laere 13 In a systematic review,Reference Burneo, Poon, Kellett and Snead 11 PET demonstrated 56-90% agreement with seizure onset localized by iEEG, and the sensitivity increased to 70-90% in those with temporal lobe hypometabolism. There is less information available regarding the usefulness of PET in eTLE, but it appears somewhat less sensitive in these cases.Reference Knowlton 14 An exception may be patients with cortical dysplasia, in whom the reported sensitivity of PET varies between 60 and 92%.Reference Lerner, Salamon and Hauptman 15 The sensitivity increased by 8-23% when PET results were combined with MRI or EEG.Reference Burneo, Poon, Kellett and Snead 11
In our study, 26 patients had discordant PET and EEG results. There are several factors that might explain these findings. First, the area of hypometabolism typically extends beyond the epileptogenic zone, making it less useful for precise neuroanatomic localization.Reference Knowlton 14 Second, other non-epilepsy factors can cause regional hypometabolism, such as preexisting parenchymal injury (gliosis and encephalomalacia) from infarcts, trauma, infections, and neurodegeneration (e.g., Alzheimer’s disease). Focal seizures that quickly secondarily generalize may have reduced sensitivity. Finally, the epileptogenic focus may be too small to be detected by PET.Reference Goffin, Dedeurwaerdere, Van Laere and Van Paesschen 16
The main limitation of our study resides in selection bias, as only potential surgical patients underwent PET. In addition, investigators were not blinded to the results of all the investigations prior to PET reporting. However, these two issues reflect what occurs in “real-life” scenarios, and they do not minimize the value of PET.
Finally, FDG was the PET tracer used in this study. The use of other tracers (e.g., 11C-flumazenil, [18F]fluoroethyl-L-tyrosine, α-methyltryptophan, and serotonin agonists) is under investigation and may improve the sensitivity and specificity of PET for presurgical localization.Reference Fedi, Reutens and Okazawa 17
In summary, PET has an important role to play in presurgical evaluation of patients with refractory epilepsy. In selected cases, it may allow resection of the epileptogenic focus without the need for iEEG. PET may also help guide intracranial electrode placement for further localization of the epileptogenic focus. In addition, it may aid in detection of multiple epileptogenic foci, thereby identifying patients as ineligible for surgical resection, and thus avoiding the need for invasive intracranial electrode studies.
Disclosures
Jorge G. Burneo reports personal fees from UCB Canada for speaker bureau work and grants from UCB Canada, outside the submitted work.
David A. Steven, Tommy L.H. Chan, and Jonathan Romsa have no conflicts of interest to disclose.





