Highlights
-
Most INO patients are orthotropic. Exotropic INO consists of slow hypometric adducting saccades, exotropia and, when unilateral, ipsilateral hypertropia.
-
Exotropic INO is due to damage of burst-tonic fibers in the MLF; orthotropic INO requires more extensive damage to extrafascicular pathways.
-
Exotropic INO does not require damage to the medial rectus subnucleus.
Introduction
Dr James A. Sharpe (1941–2013) was one of the foremost neurologists and neuro-ophthalmologists in Canada. Reference Ranalli and Morrow1,Reference Barton, Hershenfeld and Johnston2 This manuscript was in preparation at the time of his death, and 10 years after his passing, it has been completed in his honor. Internuclear ophthalmoplegia (INO) consists of limitation or slowing of adducting saccades or both due to a lesion of the ipsilateral medial longitudinal fasciculus (MLF), usually in association with jerk nystagmus of the abducting eye. Reference Hoyt, Daroff, Bach-y-Rita and Collins3–Reference Leigh and Zee6 The eyes typically remain aligned (orthotropic), maintaining binocular fusion during forward gaze. If unilateral, the patient may note vertical diplopia from disruption of otolith pathways traveling in the MLF; this causes skew deviation in 50% of patients with unilateral INO due to hypertropia of the eye ipsilateral to the adduction paresis. Reference Zwergal, Cnyrim and Arbusow7
Wall-eyed bilateral internuclear ophthalmoplegia (WEBINO), a term attributed to Martin Lubow by Hoyt and Daroff Reference Hoyt, Daroff, Bach-y-Rita and Collins3 in 1971, is a variant of bilateral INO characterized by exotropia in the primary position, resulting in horizontal diplopia. The exotropia was originally thought to be due to the involvement of the medial rectus subnuclei. Reference Hoyt, Daroff, Bach-y-Rita and Collins3 However, in 1924, Spiller Reference Spiller5 reported a patient with WEBINO and preserved convergence in whom neuropathological examination showed infarction of both MLFs in the midbrain, sparing the oculomotor nuclei. In 1979, Dr Sharpe examined a 51-year-old man presenting with acute horizontal diplopia (Patient 1), who had left INO and primary position left exotropia, presumed to be due to a brainstem stroke. In his notes, Sharpe coined the term wall-eyed monocular internuclear ophthalmoplegia (WEMINO) to describe this patient’s findings. However, a review of the literature revealed descriptions of three previous cases with clinical features consistent with WEMINO. In 1950, Cogan et al. Reference Cogan, Kubik and Smith8 reported one case of WEMINO, and in 1956, Fine and MacGlashan Reference Fine and MacGlashan9 reported two further cases. None of these had oculographic confirmation of the INO. Since we described four cases of WEMINO in 1994, Reference Johnston and Sharpe10 six other cases have been published with radiological localization of the causative lesions in the pontine tegmentum Reference Ikeda and Okamoto11–Reference Chandran, Balakrishnan, Umakanthan and Govindarajan15 or at the ponto-mesencephalic junction. Reference Shaikh, Ghasia, Rasouli, DeGeorgia and Sundararajan16
We describe the oculographic features of six patients with the clinical manifestations of WEMINO, along with neuropathological correlation in a seventh case. Six orthotropic patients with INO were also assessed quantitatively for comparison with the exotropic INO patients. A proposed hypothesis for exotropic INO syndromes initiated by Dr Sharpe and revised in accordance with more recent literature is presented.
Patients and methods
We examined seven patients with WEMINO consisting of unilateral INO and primary position exotropia (mean age 46; SD 16) and six orthotropic patients with unilateral or bilateral INO (mean age 51; SD 16) (Table 1). Patients were initially distinguished as WEMINO based on clinical assessment by one or both of the authors. Four of the seven WEMINO patients and two of the six orthotropic patients suffered brainstem infarcts. Figure 1A is an MRI of the right pontine tegmentum stroke suffered by Patient 2 (arrow). Demyelinating disease occurred in two of the WEMINO patients and four of the orthotropic patients. The MRI scan of the right pontine demyelinating lesion from Patient 3 is shown in Figure 1B (arrow). Five of six orthotropic patients had bilateral INO. Of the WEMINO patients, three had ipsilateral hypertropia. Only one WEMINO patient had exotropia contralateral to the INO. Of the six surviving exotropic INO patients, three had complete resolution of the INO and exotropia (Patients 1, 3 and 6), with one patient showing partial resolution of both INO and exotropia (Patient 5). Of the orthotropic patients, only Patient 2 showed resolution of the INO. Student’s t-test was used to compare differences between exotropic and orthotropic saccade and vestibulo-ocular reflex (VOR) metrics. For all statistical analyses, a two-sided value of p ≤ 0.05 was considered significant. Statistical analysis was performed with IBM SPSS, version 28.
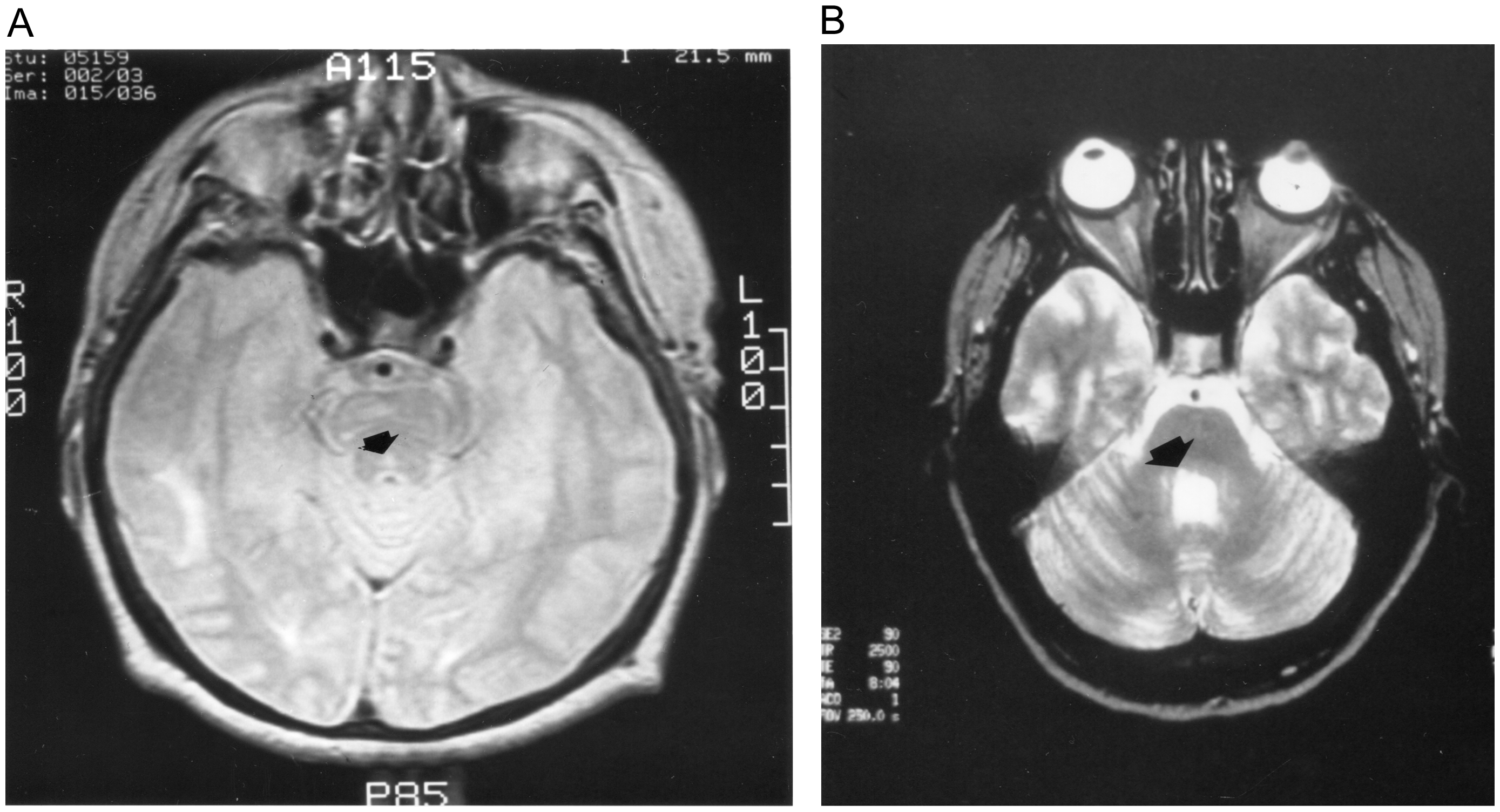
Figure 1. Neuroimaging of exotropic INO patients. (A) MRI scan of Patient 2 shows small hyperintensity in the right pontine tegmentum. (B) MRI of Patient 3 shows T2 hyperintensity in right pontine tegmentum.
Table 1. Demographics
Oculographic studies
Binocular eye and head movement recordings were undertaken using a magnetic search coil technique (CNC Engineering, Seattle WA) in five of seven WEMINO patients and all six orthotropic patients with INO. Reference Johnston, Miller and Nath17 Patient 1 was recorded using infrared oculography. Reference Sharpe and Zackon18 Informed consent was obtained from all patients and control subjects. The study protocol was approved by the University Health Network and University of Manitoba Ethics Committees and conformed to the Declaration of Helsinki. Five of six WEMINO patients and all orthotropic INO patients were recorded within one week of presentation. The exotropic eye was patched during the recording so that the non-paretic eye viewed the target, while movements of both eyes were recorded simultaneously. Orthotropic INO patients were not patched. Patients sat in a vestibular chair with the head secured by occipital and brow supports. Horizontal saccades were made to a laser dot target that subtended 0.25 deg and made predictable steps of 10 or 20 degrees and unpredictable steps of 5, 10, 20 and 40 degrees to the right or left of midposition at 3-sec intervals. Horizontal VOR was assessed during sinusoidal en bloc head and body rotation at 0.5 Hz (± 10 degrees to either side of midposition) in darkness, without a fixation target.
Analogue signals of the target, gaze (eye-in-space) and head position were digitized online at 200 Hz and analyzed by interactive computer programs as previously described. Reference Johnston, Miller and Nath17 Eye velocity signals were obtained by digitally differentiating eye position signals. Saccades were identified as previously described. Reference Johnston, Miller and Nath17,Reference Sharpe and Zackon18 Peak velocities and amplitudes of individual saccades were used to calculate the asymptotic peak velocity from a best-fit exponential curve (PV = V [1–exp (−A/C)], where PV is peak velocity, V is the asymptotic velocity, A is saccade amplitude and C is a constant). Saccadic gain was defined as the ratio of the initial saccade amplitude to the target amplitude.
As Patient 1 was recorded with infrared oculography, INO was defined as hypometria of adducting saccades with gains less than 0.66 or slow adducting saccades with asymptotic peak velocities less than 305 deg/s. Reference Sharpe and Zackon18 For the remaining 11 patients recorded with scleral search coil, INO was defined as hypometria of adducting saccades with gains less than 0.89 or slow adducting saccades with asymptotic peak velocities below 361 deg/s. Normative data for scleral search coil recordings were obtained from nine control subjects (mean age 34 years; SD 7).
Pathological examination
A 28-year-old patient with WEMINO was examined pathologically (Patient 7). He had been admitted in a coma after an assault, with CT showing bifrontal cerebral contusions, subarachnoid and intraparenchymal hemorrhages and occipital skull fracture. His recovery was complicated by elevated intracranial pressure, generalized seizures and hydrocephalus requiring a ventriculo-peritoneal shunt. He regained consciousness, and 10 weeks after admission, he complained of horizontal diplopia. Examination revealed normal visual acuity, pupils and fundi. He had right homonymous superior quadrantanopsia. In the primary position, he fixated with the left eye and showed right exotropia and right hypertropia in the primary position with complete right INO.
Four months after admission, he died of aspiration pneumonia. Necropsy revealed contusions of both frontal and temporal lobes and hemorrhagic infarction of the inferomedial left temporal and occipital lobes. Histological examination of the upper midbrain showed focal infarcts, lateral to the right MLF; the right oculomotor nerve nucleus was not involved (Figure 2A). In the lower midbrain, infarction was located adjacent to the right MLF and involved the brachium conjunctivum (Figure 2B,C). Sections through upper-mid-pons showed infarction of the right MLF (Figure 2D). There was no involvement of pontine tegmentum left of midline. Oculography could not be performed prior to this patient’s death.
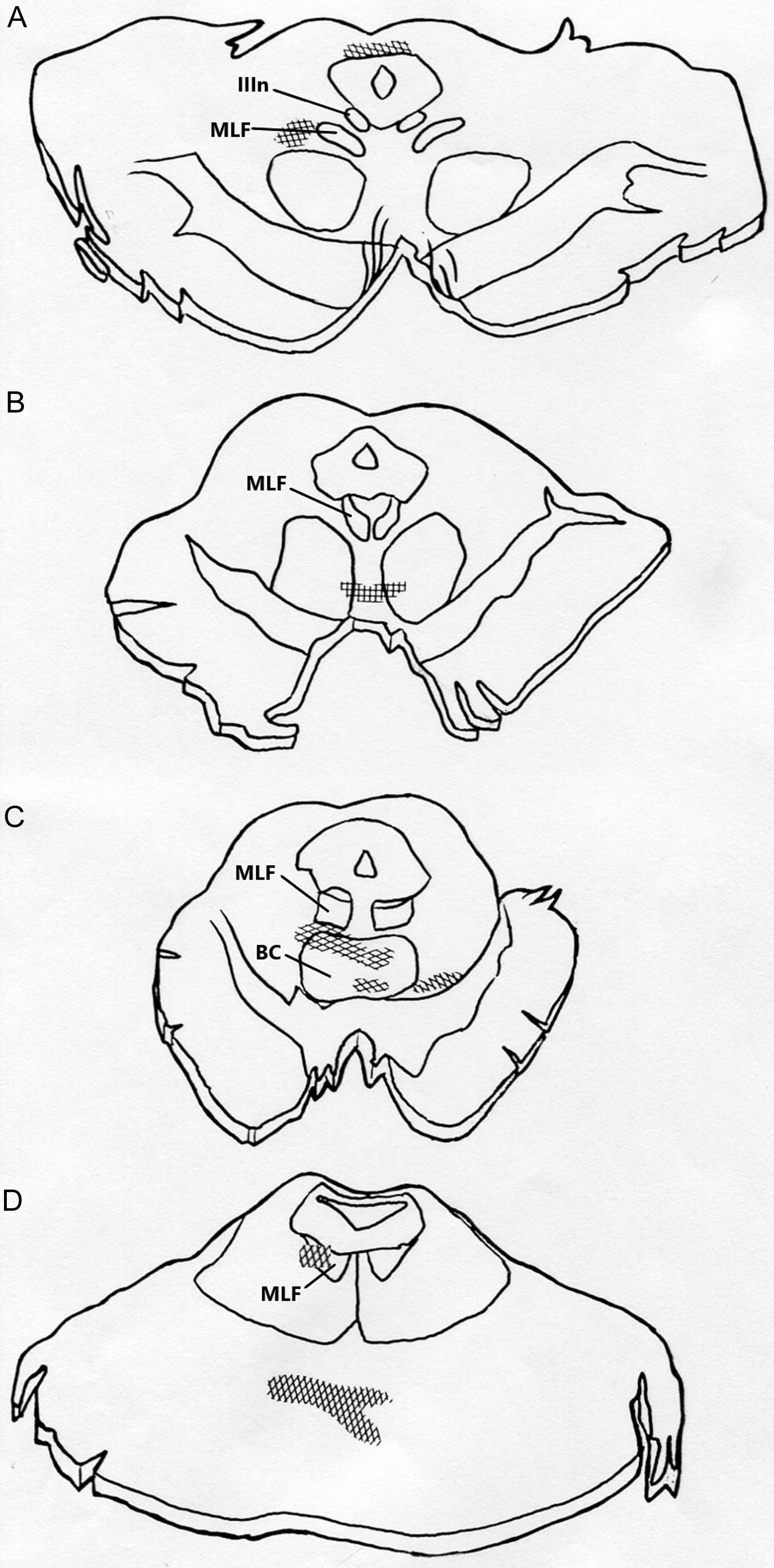
Figure 2. Histological examination of Patient 7’s brainstem; hatched areas show regions of infarction. (A) Upper midbrain shows focal infarcts lateral to the right MLF and dorsal to the periaqueductal gray matter with no involvement of either oculomotor nerve nuclei. (B and C) Lower midbrain cuts show infarction adjacent to the right MLF and involving brachium conjunctivum (BC). (D) Upper to mid-pons shows infarction of the right MLF and basis pontis.
Results
Saccade metrics
On clinical examination, all seven patients with WEMINO were exotropic, with unilaterally hypometric and slow adducting saccades and abducting nystagmus of the fellow eye. None showed abnormalities of ductions in the fellow eye clinically. Quantitatively, all exotropic patients showed slow, hypometric adducting saccades ipsilateral to the INO when compared with velocities and gains of normal horizontal saccades (Table 2).
Table 2. Horizontal saccade gains and velocities
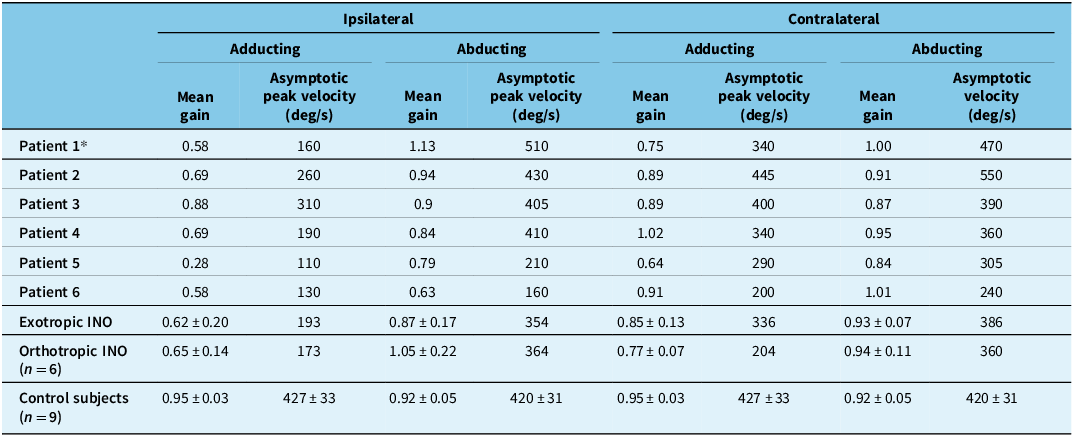
* Patient recorded with infrared oculography. Normal mean gain 0.86 ± 0.10; normal asymptotic peak velocity 495 ± 95 deg/sec. Reference Sharpe and Zackon18
There was no significant difference in mean gains or asymptotic peak velocities of ipsilateral adducting saccades between exotropic and orthotropic INO patients (Table 2). Both exotropic and orthotropic patients had normal peak velocities for ipsilateral adducting saccades less than 5 degrees. Saccade velocities decreased in a similar fashion for both groups of INO patients for larger ipsilateral adducting saccades, compared with control subjects (Figure 3).
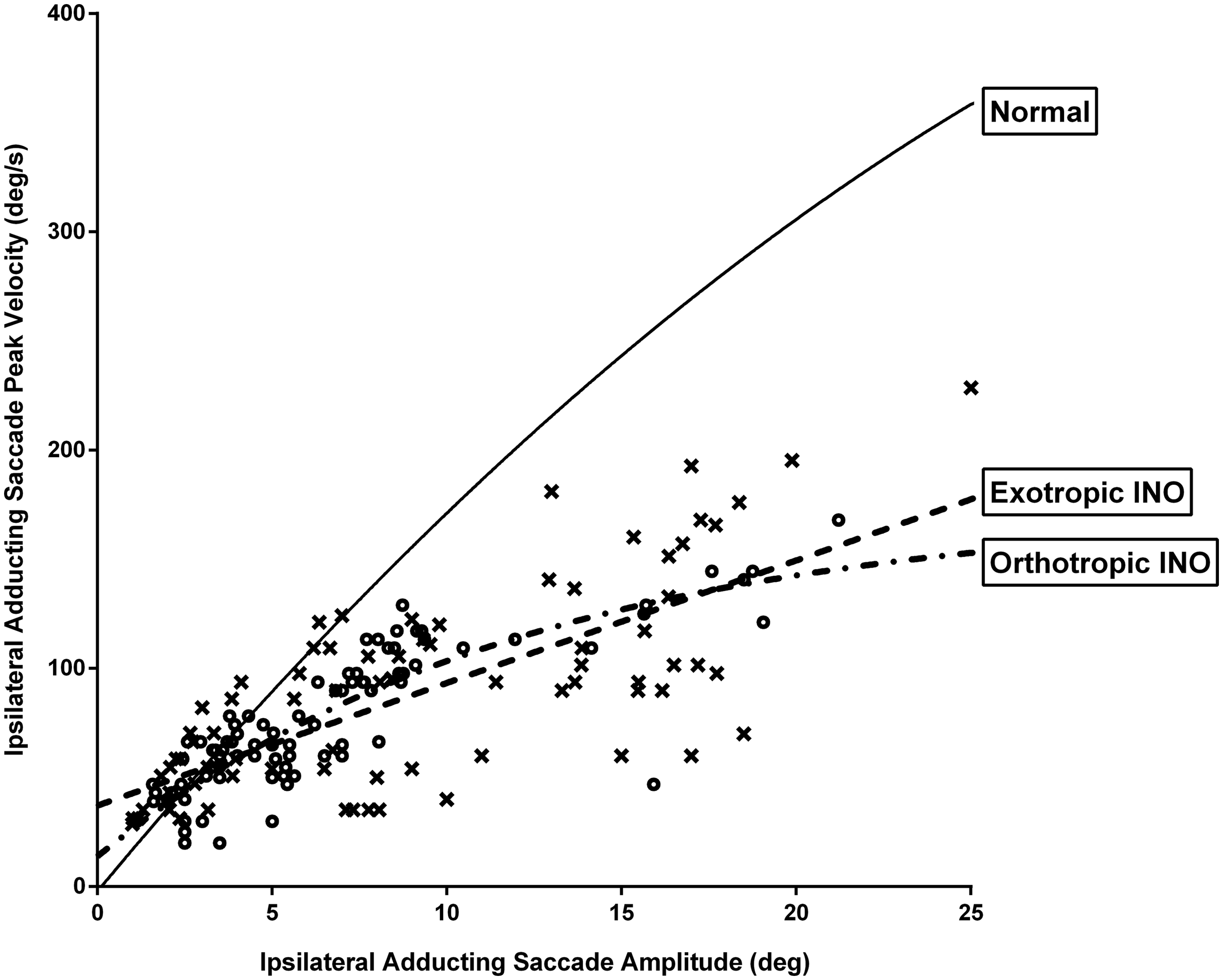
Figure 3. Graphic representation of saccade peak velocity versus saccade amplitude for ipsilateral adducting saccades for internuclear ophthalmoplegia (INO) patients versus control subjects (solid line). Both exotropic and orthotropic INO patients had normal peak velocities for ipsilateral adducting saccades less than 5 degrees and reduced peak velocities for larger ipsilateral adducting saccades compared with control subjects. x are exotropic patients; o are orthotropic patients.
For contralesional saccades, quantitative measurements of INO in clinically defined WEMINO patients showed that one patient (Patient 5) had slow, hypometric adducting saccades and two patients (Patients 4 and 6) had slow normometric adducting saccades, all consistent with bilateral INO (WEBINO). In addition to slow contralesional adducting saccades, Patient 6 also had slow, hypometric ipsilateral abducting saccades, slow contralesional abducting saccades and exotropia in the contralateral eye, more in keeping with paralytic pontine exotropia. Otherwise, ipsilateral abducting saccades were of normal asymptotic peak velocity and gain for the orthotropic group and for the remaining five exotropic patients (Table 2).
Ipsilateral abducting saccades had significantly prolonged durations in INO patients, both exotropic (171 ms, SD 10 ms, p < 0.0001) and orthotropic (190 ms, SD 34 ms, p = 0.0193) compared with control subjects (125 ms, SD 4 ms). Although INO patients would typically make ipsilateral abducting saccades of normal gain and peak velocity, they would intermittently make a series of 3–5 small, hypometric saccades in order to achieve the target (Figure 4). These smaller abducting saccades had greater than normal peak velocities (Figure 5). For orthotropic patients, 97% of 10-degree ipsilateral abducting saccades had velocities greater than two standard deviations above the mean for control subjects (193 deg/sec), compared with 48% of saccades for exotropic patients. These saccades all met the oculographic definition of saccades based on velocity and duration criteria Reference Johnston, Miller and Nath17 and may represent “fractionation” of larger abducting saccades, as described by Feldon et al. Reference Feldon, Hoyt and Stark19
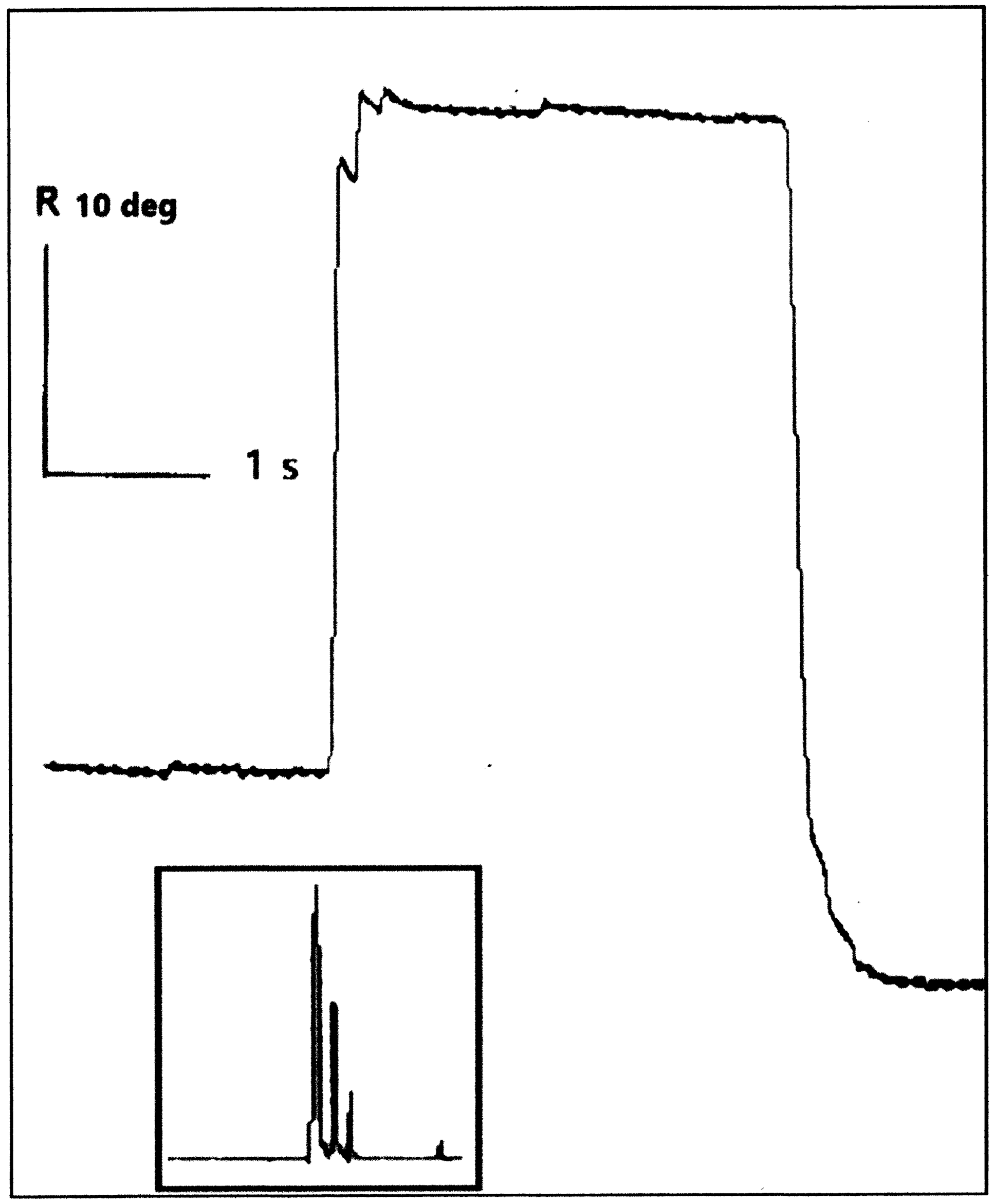
Figure 4. Ocular motor scleral search coil gaze position recording of the right eye of Patient 2 with right internuclear ophthalmoplegia. Rightward saccades are ipsilateral abducting saccades showing a series of four small, hypometric saccades for a 20-degree center-crossing target. Insert shows velocity tracing for the same saccade indicating multiple saccades used to acquire target.
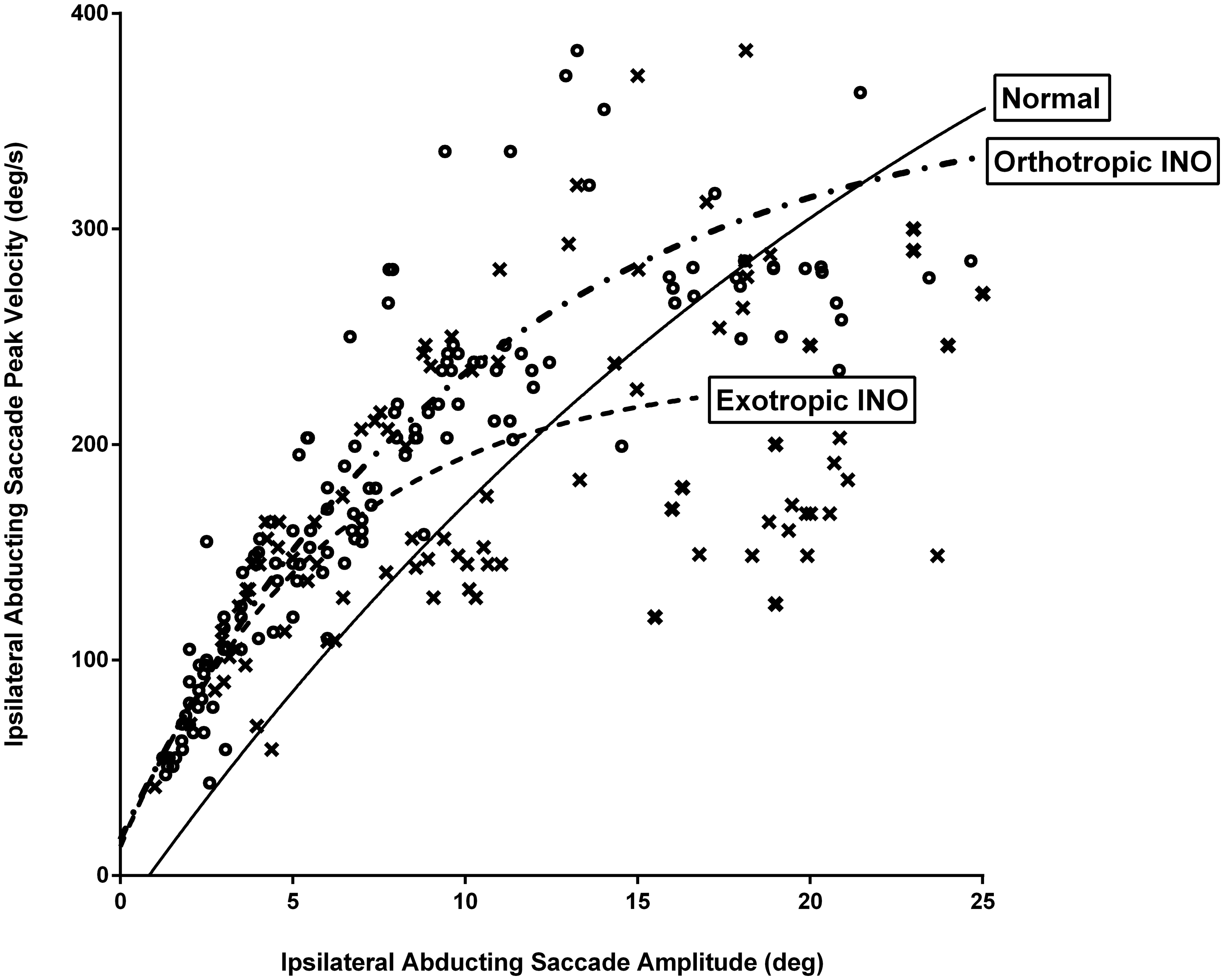
Figure 5. Graphic representation of saccade peak velocity versus saccade amplitude for ipsilateral abducting saccades for internuclear ophthalmoplegia (INO) patients versus control subjects (solid line). For orthotropic patients, 97% of 10-degree ipsilateral abducting saccades had velocities greater than two standard deviations above the mean for control subjects (193 deg/sec), compared with 48% of ipsilateral abducting saccades for exotropic patients. x are exotropic patients; o are orthotropic patients.
Horizontal vestibulo-ocular reflex (VOR)
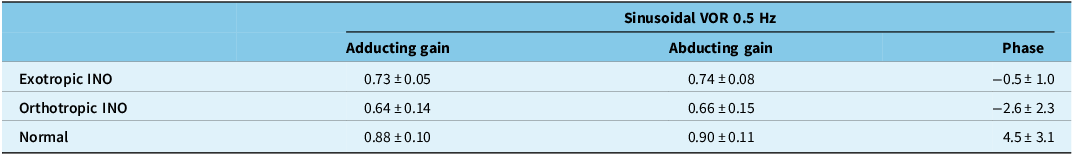
Exotropic INO patients had normal mean VOR gains during sinusoidal head rotation at 0.5 Hz for both adduction and abduction, as measured in the eye ipsilateral to the INO (Table 3). Orthotropic INO patients had subnormal mean VOR gains for both abduction and adduction. Orthotropic patients had greater phase leads than exotropic patients, but there were no statistically significant differences between groups.
Table 3. Horizontal vestibulo-ocular reflex (VOR) gains and phases
Discussion
Dr Sharpe coined the term WEMINO in 1979 when he examined Patient 1. He defined it as a “genuine prenuclear disorder” consisting of unilateral INO and exotropia. Reference Johnston and Sharpe10 In WEMINO, the exotropic eye is ipsilateral to the side of the MLF damage, and there is no gaze palsy, unlike paralytic pontine exotropia, which consists of unilateral pontine gaze palsy and INO. Paralytic pontine exotropia is a common element of the one-and-a-half syndrome due to damage to the paramedian pontine reticular formation (PPRF) or abducens nucleus and MLF on the same side and producing exotropia of the eye opposite to the side of the unilateral brainstem lesion. Reference Sharpe, Rosenberg, Hoyt and Daroff20 This would explain the findings in Patient 6, whose exotropia was contralateral to the INO.
Despite having the characteristic appearance of WEMINO clinically, three of the six patients who underwent oculography showed varying degrees of abnormal adduction of the contralesional eye, thus having WEBINO. Two measures of versional dysconjugacy indices Reference Frohman, Frohman and O’Suilleabhain21,Reference Nij Bijvank, van Rijn, Balk, Tan, Uitdehaag and Petzold22 were also calculated for these patients; only one patient (Patient 4) was classified as having bilateral INO by these indices.
Anatomy
Four patients had radiological or neuropathological evidence of isolated damage to the MLF in the pontine tegmentum (Patients 2, 3, 6 and 7). We cannot be certain that the right medial rectus subnucleus was undamaged in Patient 5, but the failure of right eye adduction in this patient was accompanied by abducting nystagmus in the fellow eye, in keeping with INO, not MR palsy. Reference Al-Sofiani and Kwen23 Although oculomotor motor neurons have been found among the fascicles of the MLF, about 1 mm lateral to the oculomotor nucleus, Reference Buttner-Ennever and Akert24 no other patient had involvement likely to include the third nuclear complex.
Of special historical relevance is the first pathological correlation of exotropia and INO that specified damage to the MLF. In 1924, Spiller Reference Spiller5 described a patient with bilateral INO, “marked divergent strabismus” and preserved convergence, resulting from infarction of both MLFs at the level of the trochlear nuclei, sparing the oculomotor nuclei. Gonyea Reference Gonyea25 described a WEBINO case with absent convergence, due to infarction of both MLFs in the pons, again sparing the oculomotor nuclei. McGettrick and Eustace Reference McGettrick and Eustace26 attributed the WEBINO syndrome to lesions affecting the PPRF and MLF but provided no radiological or neuropathological support for their speculation. Further cases of WEBINO have been reported with radiological confirmation of damage to the pons or midbrain (see ref 27, 28 for review). Damage to the medial rectus subnucleus of the oculomotor complex is clearly not required to produce exotropia. While we do not know whether our patients had preexisting heterotropias, in those whose INO resolved, their exotropia also resolved, suggesting a common mechanism.
Exotropia and saccade metrics
According to Dr Sharpe, one of the enigmas regarding INO is the typical orthotropia during forward gaze. Surgical lesions of both MLFs in monkey pons Reference Evinger, Fuchs and Baker29,Reference de Jong, Cohen, Matsuo and Uemura30 produce exotropia with preserved convergence, while chemical damage to one MLF produces exophoria of the ipsilateral eye that increases with the degree of dysfunction. Reference Gamlin, Gnadt and Mays31 Horizontal burst-tonic fibers travel within the medial portion of the MLF, arising from the contralateral abducens nucleus and projecting to the ipsilateral medial rectus subnucleus. Reference Pola and Robinson32 These fibers discharge with bursts before and during saccades in the on-direction and fire tonically at rates linearly related to eccentric horizontal eye position during fixation. Reference Pola and Robinson32,Reference King, Lisberger and Fuchs33 Burst-tonic MLF fiber activation occurs over a large range, beginning as far as 65 degrees in the off direction, Reference King, Lisberger and Fuchs33 which could account for exotropia with isolated MLF lesions.
In our study, all INO patients had ipsilateral abducting saccades with increased peak velocities for saccades of under 10 degrees amplitude. In monkeys, discrete chemical lesions of the MLF cause hypometric abducting saccades with increased peak velocities. Reference Gamlin, Gnadt and Mays31 Feldon et al. Reference Feldon, Hoyt and Stark19 showed normometric, slightly slowed ipsilateral abducting saccades with prolonged durations, similar to our patients. These saccades were “fractionated,” and their small components had higher than normal velocities. This phenomenon was attributed to deficient ipsilateral medial rectus inhibition. Reference Feldon, Hoyt and Stark19 Normally, the ipsilateral medial rectus is switched off during contralateral saccades, but intermittent braking exerted on abducting saccades by unopposed inhibitory signals produces saccades with prolonged duration, normal amplitude and velocity and faster small subcomponents, similar to those seen in our INO patients. Reference Feldon, Hoyt and Stark19 Inhibitory signals to medial rectus motor neurons project from the ipsilateral pontine reticular formation, Reference Highstein, Cohen and Matsinami34,Reference Grantyn, Grantyn, Gaunitz and Robine35 likely coursing ventral to the MLF in the pontine tegmentum, Reference Buttner-Ennever, Miles and Henn36,Reference Buttner-Ennever and Henn37 but inhibitory signals to the medial rectus subnucleus have not been recorded in the MLF of monkeys. Reference Pola and Robinson32 Thomke et al. Reference Thomke, Hopf and Kramer38 proposed “abduction paresis of prenuclear origin” resulting from damage to an uncrossed pathway ascending adjacent to the MLF that carries inhibitory fibers to the medial rectus subnucleus. Their patients were typically orthophoric or esotropic and had slowing of large abducting saccades (>20–40 degrees); smaller saccade amplitudes were not tested. Reference Thomke, Hopf and Kramer38 Localization of the lesions in their patient cohort was undertaken with electrophysiological studies and was not verified by neuroimaging. The abduction paresis was attributed to impaired inhibition of the tonic activity of the medial rectus muscle during lateral gaze. On the basis of these studies, if tonic inhibition of the ipsilateral medial rectus motor neurons is lost, orthophoria or esotropia results, and small ipsilateral abducting saccades have longer durations and higher than normal velocities. While almost all small ipsilateral abducting saccades in our orthotropic group had increased peak velocities, fewer ipsilateral abducting saccades had increased peak velocities in the exotropic group, suggesting greater maintenance of tonic ipsilateral medial rectus inhibition.
Pola and Robinson Reference Pola and Robinson39 postulated that the eyes usually remain horizontally aligned in INO because a lesion of one MLF causes decreased excitation of the ipsilateral medial rectus and decreased inhibition of the contralateral medial rectus, deviating the eyes conjugately toward the side of the MLF lesion. The brain adjusts for this deviation with an equal and opposite conjugate command, keeping the eyes orthotropic. However, there has been no anatomical confirmation of a crossed inhibitory pathway to the contralateral medial rectus subnucleus.
Vestibular function
Exotropic INO patients had normal mean VOR gains during sinusoidal en bloc head rotation for both adduction and abduction, as measured in the eye ipsilateral to the INO, compared with orthotropic INO patients who showed subnormal mean VOR gains for both abduction and adduction. Aw et al. Reference Aw, Chen, Todd, Barnett and Halmagyi40 measured vestibular function in orthotropic INO patients with head impulse testing. Their testing showed reduced horizontal VOR gains for adducting compared with abducting eye movements. Using slower impulse vestibular stimulation, we had previously shown similar, but symmetrical reductions in INO patients. Reference Sharpe, Johnston, Sharpe and Barber41 VOR latency was not prolonged, and we concluded that the direct VOR pathway in the MLF was intact enough to initiate a response, without delay, albeit at much lower gain. Steady-state sinusoidal VOR gains were normal, suggesting integrity of vestibular pathways carrying integrated position signals outside of the MLF, such as the Ascending Tract of Dieters (ATD). The ATD is an uncrossed excitatory ocular motor pathway arising from the ventral part of the lateral vestibular nucleus, passing through the ipsilateral abducens nucleus without synapsing and coursing lateral to the MLF, to excite ipsilateral medial rectus motor neurons, inferior rectus motor neurons and the Edinger–Westphal complex. Reference Reisine, Strassman and Highstein42–Reference Zwergal, Strupp, Brandt and Buttner-Ennever44 Axons of the ATD excite the medial rectus during ipsilateral angular and linear head movement. Reference Reisine, Strassman and Highstein42,Reference Chen-Huang and McCrea45 In our cohort of orthotropic patients, VOR gains were subnormal and showed phase leads, suggesting damage to indirect, extrafascicular VOR pathways carrying integrated vestibular eye position signals. Reference Sharpe, Johnston, Sharpe and Barber41
Conclusion
WEMINO is an uncommon clinical variant of INO described by Dr J. A. Sharpe initially in 1979. It is best defined as a clinical ocular motor syndrome characterized by unilateral slow or hypometric adducting saccades or both, with exotropia and hypertropia of the ipsilateral eye.
Common to both orthotropic and exotropic INO patients is a lesion in the MLF that damages burst-tonic fibers carrying velocity and position commands to medial rectus motor neurons, causing ipsilateral paresis of adduction. There is a clear overlap with WEBINO, which can be explained by extension of damage to include the contralateral MLF. Damage to direct VOR pathways transmitted through the MLF may reduce initial VOR gains in all INO patients; however, steady-state VOR gains are normal in exotropic patients and subnormal in orthotropic patients, with greater phase leads suggesting damage to indirect VOR pathways outside the MLF in orthotropic patients. Finally, the loss of tonic inhibition of ipsilateral medial rectus motor neurons results in orthotropia, longer duration ipsilateral abducting saccades and small, possibly “fractionated” ipsilateral abducting saccades with increased peak velocities. While small, fast ipsilateral abducting saccades occurred in both orthotropic and exotropic cohorts, fewer ipsilateral abducting saccades had increased peak velocities in the exotropic group, suggesting greater maintenance of tonic ipsilateral medial rectus inhibition. Inhibitory signals to the medial rectus do not travel in the MLF but likely ascend ventral to the MLF in the pontine tegmentum. Therefore, orthotropia requires paramedian damage to extend beyond the MLF, ventrally and laterally, involving integrated vestibular position signals in the ATD and inhibitory connections to the ipsilateral medial rectus subnucleus. Exotropic INO patients (both WEMINO and WEBINO) may have more discrete damage to the MLF with limited involvement of extrafascicular pathways. In neither case is damage to medial rectus motor neurons required.
Acknowledgments
Dr Johnston would like to acknowledge the kind assistance of Dr Mark J. Morrow in the preparation of this manuscript.
Author contributions
JJ and JS conceived the study. JJ and JS examined the patients and undertook the oculography. JJ and JS wrote and edited the manuscript.
Funding statement
None.
Competing interests
None.










