Introduction
Neuropsychologists often work in a clinical setting with older adults, assessing and providing interventions for neurocognitive disorders such as mild cognitive impairment and dementia. These clinicians must be aware of potential co-morbidities in their clients which could affect service provision. Co-morbidities are especially important to consider in an older population, as the majority of individuals over the age of 65 years will present with at least two chronic health conditions (Salive, Reference Salive2013). Hearing loss is the third most common chronic health condition in older adults, after arthritis and heart disease, with prevalence rates estimated at 50 per cent for individuals over the age of 65 years, and 90 per cent for those over the age of 80 years (Bainbridge & Wallhagen, Reference Bainbridge and Wallhagen2014; Cruickshanks et al., Reference Cruickshanks, Wiley, Tweed, Klein, Klein, Mares-Perlman and Nondahl1998). Unfortunately, treatment rates of hearing loss in older adults are very low, with less than a quarter of individuals who need hearing aids actually purchasing them (Kochkin, Reference Kochkin2005). The average age of first-time hearing aid users is 63 years old (Abrams & Kihm, Reference Abrams and Kihm2015), and the average time an individual will wait between acknowledging hearing loss and seeking treatment is 10 years (Davis, Smith, Ferguson, Stephens, & Gianopoulos, Reference Davis, Smith, Ferguson, Stephens and Gianopoulos2007). Given these statistics, it is likely that many of the older clients seeking assessment and intervention for neurocognitive issues are likely to have undiagnosed and/or untreated hearing loss.
A growing body of research shows a strong link between hearing loss and the development of incident dementia in an older population (e.g., Gurgel et al., Reference Gurgel, Ward, Schwartz, Norton, Foster and Tschanz2014; Lin et al., Reference Lin, Metter, O’Brien, Resnick, Zonderman and Ferrucci2011; Yuan, Sun, Sang, Pham, & Kong, Reference Yuan, Sun, Sang, Pham and Kong2018). Although the specific causal mechanisms behind this link have yet to be determined, changes to the physical structure of the brain in those older adults with hearing loss may play a role. Indeed, accelerated whole brain and right temporal lobe volume declines have been shown in older adults with hearing loss compared to their normal-hearing counterparts (e.g., Lin et al., Reference Lin, Ferrucci, An, Goh, Doshi, Metter and Resnick2014), and Rigters et al. (Reference Rigters, Bos, Metselaar, Roshchupkin, de Jong, Robert and Goedegebure2017) have demonstrated that, independent of cognitive status and cardiovascular risk factors, hearing loss is associated with smaller total brain volume. Another possible explanation is that older adults with hearing loss are at a higher risk of social isolation (e.g., Mick, Kawachi, & Lin, Reference Mick, Kawachi and Lin2014), which in turn is a known risk factor for the development of cognitive impairment (e.g., Saczynski et al., Reference Saczynski, Pfeifer, Masaki, Korf, Laurin, White and Launer2006). Regardless of the mechanism, the data are compelling and suggest that those who are tasked with detecting cognitive impairment in an older population (e.g., physicians, neuropsychologists) must be aware of this important connection.
Routine neuropsychological practice involves assessment tools which require clients to correctly hear test items. However, if an item is misperceived during the learning phase of a memory test (e.g., client repeats “wave” rather than “cave”), or the wrong number on a digit span test is repeated, test scores will be inappropriately low, and the client may appear more impaired than they really are (Dupuis et al., Reference Dupuis, Pichora-Fuller, Chasteen, Marchuk, Singh and Smith2015). Indeed, recent work has demonstrated that reduced audibility of test materials reduces performance on commonly used screening tools in both younger and older adults (Dupuis, Marchuk, & Pichora-Fuller, Reference Dupuis, Lemke, Reed and Pichora-Fuller2016; Jorgensen, Palmer, Pratt, Erickson, & Moncrieff, Reference Jorgensen, Palmer, Pratt, Erickson and Moncrieff2016).
A client who presents with what appears to be hearing loss (e.g., many instances of repetition, says “what” or “pardon me” repeatedly during an assessment) may in fact be experiencing hearing loss related to high levels of cerumen (ear wax), with research showing that cerumen management can lead to improvement in scores on cognitive testing (e.g., Moore, Voytas, Kowalski, & Maddens, Reference Moore, Voytas, Kowalski and Maddens2002; Sugiura et al., Reference Sugiura, Yasue, Sakurai, Sumigaki, Uchida, Nakashima and Toba2014), and to significant improvement in hearing for 10 per cent of individuals presenting with hearing issues (Allen et al., Reference Allen, Burns, Newton, Hickson, Ramsden, Rogers and Morris2003). Hearing loss can even prevent standardized neuropsychological tests from being administered properly (Lin et al., Reference Lin, Chung, Callahan, Smith, Gritters, Chen and Masellis2017) and is associated with poorer scores on standardized screening measures for cognitive loss (Lim & Loo, Reference Lim and Loo2018). For this reason, it is crucial that neuropsychologists take their clients’ hearing ability into account. Unfortunately, recent research suggests that the majority of clients seen by physicians in a primary care clinic for diagnosis of dementia are not asked about their hearing (Jorgensen, Palmer, & Fischer, Reference Jorgensen, Palmer and Fischer2014), and few are referred for audiologic care. Therefore, referral documents are unlikely to provide information about the client’s hearing status. In addition, there is no evidence to suggest that neuropsychologists routinely assess their client’s hearing, and it is unclear what the formal role of the test administrator is in terms of providing hearing amplification during neuropsychological test administration (Goman, Reed, Price, & Lin, Reference Goman, Reed, Price and Lin2016).
Neuropsychologists may not be comfortable asking about a client’s hearing, or they may not know what questions to ask. In a recent systematic review, Goman et al. (Reference Goman, Reed, Price and Lin2016) failed to find any published references in which hearing ability was assessed either before or after neuropsychological evaluation, which could suggest that there was an assumption on the part of the testers that individuals could hear adequately enough to complete the testing. There is no existing literature on whether or how information about a client’s hearing status is collected as standard practice, and whether this information could influence clinical practice in neuropsychology.
The current study was a small feasibility study that examined whether on-site hearing screening would influence neuropsychologists’ practice with older clients. The results from this feasibility study will serve to inform the development of future research in this clinic, and will contribute to an important dialogue around considering hearing loss in neuropsychological practice.
Current Study
The goals of the current study were to determine (1) the objective hearing status of clients in a geriatric neuropsychology clinic through use of hearing screening tools; (2) how well neuropsychologists were able to detect hearing loss in these clients; and (3) whether possessing objective knowledge of their client’s hearing status would influence the neuropsychologists’ approach to assessment, diagnosis, treatment recommendations, and/or communication with clients.
Method
Participants
Over the course of four months in 2015, 20 individuals who were clients of a geriatric neuropsychology clinic in Toronto, Canada, were approached by a research assistant (RA) to participate in the current study. This was a convenience sample based on the availability of the part-time RA. The clinic secretary would inform the RA that a potential participant (i.e., a client who would be completing an assessment with one of the four treating neuropsychologists who participated in the study: SV, KM, KS, DR) was booked for a certain day and time. The RA would speak with the client either before or after their neuropsychological assessment to tell them about the study and inquire whether they were interested in participating. Participants provided informed consent to the RA and were each tested individually in one session lasting approximately 30 minutes following completion of the neuropsychological assessment. The treating neuropsychologists were not involved in the recruitment process.
All 20 individuals who were approached by the RA agreed to participate (mean age = 70.7 years, range = 41–88 years, 45% female). Information about first language learned was available for 18/20 participants. For 11/18 participants (61%) the first language was English alone, and for two participants it was English and Yiddish together. This work was conducted in accordance with human ethics standards and received approval from the Baycrest Research Ethics Board.
Procedure
All testing was conducted in a private, quiet room. The RA began the testing session by screening the participant’s hearing, and, for the last 11 participants, conducting a word recognition test. A measure of cognitive status was then completed, followed by a demographic questionnaire and a hearing satisfaction questionnaire.
The neuropsychologists were asked to fill out the pre-reveal questionnaire after informed consent was obtained from the client (i.e., after the neuropsychological assessment had been completed). Once the participant’s testing session was complete and the neuropsychologist completed the pre-reveal questionnaire, the researcher shared a copy of the hearing screening results with the neuropsychologist and asked them to complete the post-reveal questionnaire. The post-reveal questionnaire was returned to the RA after an average of 49 days (SD = 32.6, range = 1–129), depending on the clinician. Note that the four treating neuropsychologists interacted with their clients to (a) obtain consent, (b) conduct the clinical interview, and (c) conclude the session and explain the next clinical steps to their clients. Neuropsychological testing was conducted by psychometrists.
Measures
Montreal Cognitive Assessment (MoCA)
This test was developed to screen for mild levels of cognitive loss in an older population (Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead, Collin and Chertkow2005). It consists of 13 questions measuring the domains of visuospatial and executive function, object naming, verbal memory, attention, language, abstract thinking, and orientation. Any score lower than 26 on this measure may be indicative of cognitive loss. There was no MoCA score cut-off for inclusion in this study. The MoCA test was not administered to any of the participants during their cognitive assessment with the neuropsychologist.
Hearing Screen
We used a Siemens HearCheck Screener (https://www.connevans.co.uk/product/2831233/38SHEARCHECK/Siemens-HearCheck-Screener) to screen for hearing loss in participants, one ear at a time. The screener presents 1000 Hz tones at three sound levels (55 dBHL, 35 dBHL, 20 dBHL) and 3000 Hz tones at three sound levels (75 dBHL, 55 dBHL, 35 dBHL). The participant was asked to respond by raising their hand every time they heard a tone. The test was scored separately for each ear, and took approximately three minutes to administer. Recommendations are made by the manufacturer for each score as follows: 6 tones heard, client is unlikely to need further hearing assessment; 3–5 tones heard, client is likely to have a hearing difficulty and would benefit from hearing assessment; 1–2 tones heard, refer client for immediate assessment; 0 tones heard, refer client for an assessment at a clinic that can appropriately assess severe hearing loss. For the purposes of the present study, these ranges were termed normal, mild loss, moderate loss, and severe loss. Two participants scored below three tones in one ear; on query, both participants were aware of a pre-existing hearing loss. Any other participant, regardless of score, who reported self-perceived hearing loss was advised by the RA to speak with their general practitioner about their concerns.
Word Recognition
We used the Central Institute for the Deaf Auditory Test W-22 test (Hirsh et al., Reference Hirsh, Davis, Silverman, Reynolds, Eldeit and Benson1952) to assess word recognition and to determine aided hearing status in clients with hearing aids. The list consists of 200 words divided into eight half-lists. Each word is presented in the carrier phrase “Say the word” (e.g., “Say the word camp”). One half-list (25 words) was administered to each participant. The researcher read out each carrier phrase and word combination while ensuring that the participant could not see the researcher’s mouth in order to eliminate speech reading cues. Participants were asked to repeat the target word, or to give their best guess if they were uncertain. The researcher transcribed their responses, including any errors made. This test took approximately two minutes to complete. Score ranges for categorization were developed by the licensed audiologist based on existing norms (e.g., Schoepflin, Reference Schoepflin2012): normal range: 90–100 per cent of words repeated correctly; slight difficulty range: 76–89 per cent of words repeated correctly; moderate difficulty range: 60–75 per cent of words repeated correctly; very poor range: < 50 per cent of the words repeated correctly). For the purposes of the present study, and in an attempt to remain consistent with the results of the HearCheck Screener, these ranges were termed normal, mild loss, moderate loss, and severe loss. We amended the protocol to include this test half-way through the study; therefore, only the last 11 participants completed this test.
Questionnaires for Neuropsychologists
The neuropsychologists filled out two questionnaires relating to each participant: one pre- and one post-reveal of their client’s hearing screen results. The pre-reveal questionnaire was completed after the neuropsychologist’s encounter with the participant (i.e., in the assessment or intervention program). This questionnaire asked whether the clinician had had any anticipation of their client’s hearing status and, if so, what information they used to make this decision (e.g., medical chart, previous interaction with client). They were also asked whether their impression of their client’s hearing status changed over the course of their interaction with the client, how confident they felt about their categorization, and whether they made any modifications to their practice. The post-reveal questionnaire asked the neuropsychologists whether their opinion about their client’s hearing status had changed based on disclosure of the hearing test results. If yes, they were asked whether they would consider (a) gathering additional assessment information; (b) revising their diagnostic formulation; (c) revising their recommendations; (d) modifying their communication strategies; or (e) engaging in any other actions. They were asked to describe details of any actions taken, and had the opportunity in response option “e” to provide their own free-form response to the question.
Objective Hearing Status Classification
Consistent with audiologic best practice, participants’ hearing status was based on the performance of their better ear in the hearing screening. The hearing status of two participants who had not completed the hearing screening due to their hearing aids and/or cochlear implants, and of one participant who had completed the hearing screening after removing their hearing aid, was based upon their performance on the word recognition test. With the exception of one participant who performed slightly worse on the word recognition test than on the hearing screening, hearing status based on word recognition test scores and hearing screening results were identical (n = 10). As described above, all participants had their hearing status classified as either normal, mild loss, moderate loss, or severe loss.
Results
Participant Characteristics
Diagnosis
There were neuropsychological diagnoses available for all 20 participants. Nine participants (45%) had a diagnosis of mild cognitive impairment; five with amnestic type (one single domain, four multi-domain), three with non-amnestic type (one executive domain, one multi-domain due to Parkinson’s disease, one multi-domain with a multifactorial etiology); and one non-specified. Four participants (20%) had normal cognition for their age, three participants (15%) had a diagnosis of executive dysfunction (one participant with an unknown etiology and two due to vascular factors), and four participants (20%) had other diagnoses (one with dementia of unclear origin, one with a potential diagnosis of logopenic progressive aphasia, one with likely depression and anxiety, and one with cognitive impairment due to stroke).
Global Cognitive Status
The mean MoCA score across the 20 participants, adjusted for education, was 22.6/30 (SD = 3.8). Of the 20 participants, only 5 (25%) had scores at or above cut-off for cognitive loss (i.e., > 26).
Goal 1
Objective Hearing Status
Eighteen of the 20 participants completed the hearing screening in the left ear, and 17/20 completed the screening in the right ear. (Two participants did not complete the hearing screening in either ear due to bilateral hearing aid use and a cochlear implant, and one participant completed the hearing screening only in the left ear due to congenital deafness in the right ear.) Eleven participants completed the W-22 test of word recognition. Results are summarized in Table 1. Just over half of the participants for whom data were available (10/18 participants) demonstrated some degree of hearing loss based on better ear performance. Four participants were hearing aid users, and two of these had their hearing assessed with the word recognition test while using their aids. Interestingly, neither of these participants scored in the normal range on the test of word recognition, scoring in the slight (76% accuracy) and moderate (64% accuracy) difficulty ranges. A chi square analysis failed to reveal any difference in word recognition performance based on first language learned (p = .17). When participants were categorized into a “younger” (n = 11; ages 41–72) and an “older” age group (n = 9; ages 73–88), there was no significant difference in objective hearing status based on age (p = .24).
Table 1: Categorization of objective hearing test scores

Goal 2
Neuropsychologist Detection of Hearing Loss
The neuropsychologists rated their clients’ hearing status in all 20 cases. In the majority of the cases, the clinicians predicted that their clients would have normal hearing (9/20 participants; 45%) or mild hearing loss (10/20 participants; 50%). Mild to moderate loss was predicted only for one participant (5%), and there were no predictions of moderate or severe loss.
When compared to the objective hearing screening results, which were available for 18 participants (90%), the neuropsychologists’ ratings were accurate in the majority (12 participants; 60%) of the cases (see Table 2). There was a significant difference in the number of participants in each hearing status category (normal, mild loss, moderate loss) between the participant scores and the neuropsychologists’ ratings (χ2 = 10.12, df = 4, p = .038). The neuropsychologists were more likely to underestimate (that is, to say that a client has better hearing than was revealed by objective hearing screening; 71% of errors) hearing ability than overestimate (29% of errors).
Table 2: Neuropsychologist hearing status categorization accuracy
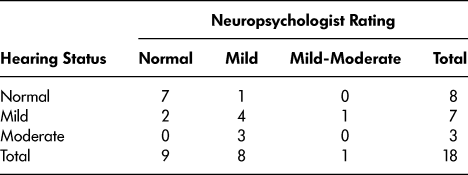
Note. Two participants who completed the hearing screening without their hearing aids were excluded, since this did not reflect their hearing status during the neuropsychological assessment. Three participants’ hearing status was based on their performance on the Central Institute for the Deaf Auditory Test W-22.
Confidence in Ratings
The clinicians were asked to estimate how confident they were in their ratings; these data were available for 19/20 participants. In 10/20 cases (50%), the clinicians said that they were very confident in their ratings, and in 9/20 cases (45%), the neuropsychologists said that they were somewhat confident. When comparing accuracy of hearing status ratings to clinicians’ confidence ratings (these data were available for 18/20 participants), the neuropsychologists were “very” confident (73%) and “somewhat” confident (27%) in their ratings in situations where there was a match between the two sets of ratings. The neuropsychologists appeared to be less confident in their judgments when they believed that the participant they were rating had poor hearing; for the cases of clients with mild loss, the neuropsychologists provided “very” confident ratings in only 33 per cent of the cases, and “somewhat” confident ratings in the other 67 per cent of the cases. In situations where there was a mismatch between the two sets of ratings, the neuropsychologists indicated they were “somewhat” confident in 6/7 cases (86%) and “very” confident in 1/7 cases (14%); they never indicated that they were “not confident” in their ratings.
Goal 3
Modifications to Clinical Practice
In five of the cases (25%), the neuropsychologist indicated that his/her understanding of their client’s hearing status changed after reviewing results from the objective hearing tests. Subsequent modifications to clinical practice fell under two categories: recommendations to clients and additional services or counselling provided. Specifically, the clinicians recommended a formal audiologic assessment (n = 3 clients), and recommended pursuing the use of a hearing assistive device (n = 1 client). They also provided clients (n = 4) and their spouses (n = 2) with education on hearing loss and communication. In three of the cases, the clinicians stated that they would provide handouts to the clients regarding hearing loss and its effects on communication if such an educational document were available.
Discussion
The purpose of this work was to conduct a small feasibility study to see whether we could successfully screen the hearing of individuals attending a geriatric neuropsychological clinic for assessment. Given (a) the important connections between hearing loss and cognitive impairment, (b) the fact that most hearing loss remains untreated in older adults, and (c) the understanding that untreated hearing loss may have a significant impact on performance on neuropsychological assessments, we also aimed to evaluate the neuropsychologists’ awareness of their clients’ hearing status and the potential impact of objective hearing screening on assessment results and treatment recommendations.
Hearing Status of Clients in a Neuropsychology Clinic
Over half of the participants in the current study who presented to a geriatric neuropsychology clinic had some degree of clinically significant hearing loss. The number of participants (56%) failing the hearing screen is consistent with population statistics of hearing loss prevalence in this age group (age range: 41–88 years). The screening was well-received by study participants, all of whom agreed to complete the study without any financial compensation. The tools administered were brief (approximately five minutes to complete), easy to administer, portable, readily available, and able to detect potential hearing loss which may have otherwise gone unnoticed by the treating neuropsychologist. These tools could be readily integrated into a routine clinical protocol prior to carrying out an assessment or intervention with an older client.
One outcome of the hearing screening was potential detection of untreated hearing loss in a minority of patients. Per study protocol, these participants were advised to seek audiologic assessment. Although it is plausible that some of these cases represent false positives, the outcomes observed present low risk of harm and considerable potential for benefit to the clients. Future study is needed to evaluate the concordance of hearing screening results with comprehensive audiologic assessment, as well as implications for treatment and health care resource utilization.
Detection of Hearing Loss by Neuropsychologists
The hearing status ratings of the clinical neuropsychologists were consistent with the objective screening data in 60 per cent of the cases; where errors occurred, these tended to be underestimations of their clients’ hearing status. It could be viewed as reassuring that the neuropsychologists were able to accurately characterize the hearing status of so many clients, and that provision of feedback regarding the objective hearing status of the clients did not impact key assessment outcomes such as revising their diagnostic formulation. This finding could speak to these neuropsychologists’ extensive experience working with an older population, many of whom likely have sensory loss. Alternately, the neuropsychologists may have been more aware of considering their patients’ hearing status by virtue of their participation in the study. Note that even these experienced neuropsychologists working in a specialized geriatric setting still underestimated hearing loss in the majority of the errors made regarding severity of hearing loss in their clients. Repeating this study in more age-diverse practice settings and/or with less experienced clinicians would be interesting avenues for future study.
Modification to Practice to Accommodate Hearing Loss
Overall, provision of feedback on the mismatch between the clinician’s judgment and the client’s objective hearing status led clinicians to change routine practice in order to address potentially untreated hearing loss (e.g., referrals), and provide education for both clients and their significant others regarding hearing health and communication. There were no reported cases of the neuropsychologist changing his/her diagnostic impressions after taking into account new information about his/her client’s hearing loss. It is possible that the neuropsychologists’ failure to revise their diagnostic formulation in some cases may represent an overreliance on experience-informed clinical judgment and a missed opportunity to integrate new information relevant to the diagnostic formulation. Further research could address these important issues.
Implications for Policy and Practice
Our preliminary findings from a small feasibility study serve as an important reminder to be vigilant in considering hearing loss as a potential contributor to the day-to-day experience of cognitive change in older adults, and suggest that routine screening for hearing loss in older clients can feasibly be integrated into standard neuropsychological practice. Neuropsychologists working with older adults can incorporate simple hearing screening questions (e.g., Have you ever had your hearing tested? Do you ever find that people are talking but you can’t make out the words? Do you have difficulty following conversations in busy environments, such as a noisy restaurant?) into their history-taking, and remain alert for signs of hearing loss (e.g., numerous requests for repetition) during client interactions. Amplification may make it easier for neuropsychologists to be confident that their clients did accurately hear auditorily presented information, and amplification devices (e.g., Pocketalker; https://www.williamssound.com/pocketalker) should be readily available and offered to clients.
Study findings also underscore the importance of considering hearing health in formulating treatment recommendations for clients. Research has linked hearing loss to social isolation (Mick et al., Reference Mick, Kawachi and Lin2014), which in turn is a risk factor for depression (Taylor, Taylor, Nguyen, & Chatters, Reference Taylor, Taylor, Nguyen and Chatters2016) as well as functional decline and death (Perissinotto, Stijacic Cenzer, & Covinsky, Reference Perissinotto, Stijacic Cenzer and Covinsky2012). Discussing these links with some clients may encourage them to sustain social engagement, with secondary benefits for mental health and overall well-being as they age. In some clients, hearing loss may be a contributor to presenting concerns of depression or anxiety, and treatment outcomes may be enhanced by explicit attention to this.
Interprofessional collaboration was essential to the study design and execution, and presumably critical to the successful launch and evaluation of any practice changes informed by study findings. Neuropsychologists should be prepared to recommend referrals for hearing care in their reports, and seek out the guidance of audiologists if they have a case where they suspect hearing loss. The present study findings complement recent work recommending parallel collaboration to audiologists working with older individuals who have medical co-morbidities, such as cognitive loss (e.g., Dupuis, Lemke, Reed, & Pichora-Fuller, Reference Dupuis, Lemke, Reed and Pichora-Fuller2016; Reed, Dupuis, & Pichora-Fuller, Reference Reed, Dupuis, Pichora-Fuller, Valente and Valentein press). Interprofessional collaboration and education to inform neuropsychologists about the signs and symptoms of hearing loss, as well as specific questions they could use to quickly identify hearing loss, may help clinicians to become more accurate and confident when determining hearing status of their clients (Dupuis, Reed, & Pichora-Fuller, Reference Dupuis, Reed and Pichora-Fuller2014).
Conclusions
This is the first study to objectively screen for hearing loss in the clients of a geriatric neuropsychology clinic. Results showed that integrating hearing screening into neuropsychological assessment practice was feasible and led to identification of clients with potentially untreated hearing loss and provision of education and referrals for such persons. As hearing health is a key factor in healthy aging, findings reinforce the importance of careful attention to clients’ sensory status in neuropsychological assessment practice and interprofessional collaboration as means to optimize validity of cognitive assessments and provide useful treatment recommendations to clients.




