Background
Canada has met a critical milestone: As of July 2016, there were a greater number of older adults than there were children under the age of 15 (Canadian Medical Association, 2016). With the increasing number of older adults comes a growing population that presents to the health care system with multiple health challenges. For example, 85 per cent of older adults are living with at least one or more chronic conditions (Patrick et al., Reference Patrick, Psych, Knoefel, Coe, Gaskowski and Rexroth2001), and 25 per cent are living with frailty (Koné Pefoyo et al., Reference Koné Pefoyo, Bronskill, Gruneir, Calzavara, Thavorn, Petrosyan and Wodchis2015). Older adults make up 40 per cent of acute hospital stays and stay in hospital 1.5 times longer than those younger than 65 years of age (Canadian Institute for Health Information Board of Directors, 2011; Canadian Medical Association, 2013). Thirty-five per cent of older adults admitted to the hospital every year experience a decline in activities of daily living (ADL) during their hospital stay, which, in turn, leads to difficulty returning and staying at home post-hospital discharge (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; Kortebein, Reference Kortebein2009). Furthermore, 8.5 per cent of older adults discharged from the hospital return to the hospital within the first 30 days post-discharge (Pathipvanich et al., Reference Pathipvanich, Tsuchiya, Rojanawiwat, Schmidt, Auwanit, Sawanpanyalert and Ariyoshi2013). Those with a greater number of co-morbidities, frailty, cognitive decline, and dementia have the highest rates of readmission (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; Kortebein, Reference Kortebein2009).
Canada’s Medicare system was developed to address acute, episodic care for a fairly independent and healthy population (Canadian Medical Association, 2016). Older adults are often unprepared for transitions home from hospital and are not always physically or emotionally able to live independently, leading to increased caregiver stress, health care expenditures, and pressure on health care providers (Bauer, Fitzgerald, Haesler, & Manfrin, Reference Bauer, Fitzgerald, Haesler and Manfrin2009). Despite the discussions and debates regarding the use of and need for transitional rehabilitation programs and continuity of care for older adults living in the community, there continues to be a gap in providing an effective and efficient continuum of health care services for older adults that will keep older adults at home and out of hospitals. This gap has occurred in part due to the lack of availability of post-acute services, such as services to address chronic illness, medication management, disability adjustment, and transitional and community care needs (Koné Pefoyo et al., Reference Koné Pefoyo, Bronskill, Gruneir, Calzavara, Thavorn, Petrosyan and Wodchis2015).
Rehabilitation for Older Adults Post-hospitalization
There are a variety of rehabilitation program models intended to assist older adults to return to pre-illness function post-hospitalization, and programs vary in practice across the provinces. For example, in Ontario an older adult needing rehabilitation, but deemed not eligible for rehabilitation in the community, may enter a complex continuing care (CCC) unit or be considered for an alternate level of care (ALC), a level of care geared for patients who are medically stable but not ready to be discharged home due to loss of ability to perform ADL (Nord, Reference Nord2009). Older adults undergoing rehabilitation in CCC or ALC tend to be frail, live alone, have multiple co-morbidities, and to be deemed to have low to no rehabilitative capacity, which is not always the case (Sutherland & Trafford Crump, Reference Sutherland and Trafford Crump2013; Walker, Morris, & Frood, Reference Walker, Morris and Frood2009).
Generally, rehabilitation programs for older adults have similar goals: to maximize functional recovery and independence post-hospitalization in a safe and cost-effective manner, and to decrease re-hospitalization (Kortebein, Reference Kortebein2009). Traditional rehabilitation programs are considered to be shorter in duration and higher in intensity (Stott & Quinn, Reference Stott and Quinn2013). In Ontario, the typical length of traditional rehabilitation programs for older adults is two to eight weeks and with rehabilitation sessions taking place five to seven days a week for 120 minutes a day. These programs are offered in the hospital (in-patient rehabilitation) or are delivered on an outpatient basis (GTA Rehab Network, 2008). Previous research has shown that traditional rehabilitation programs are beneficial for older adults transitioning from hospital to home and have a positive impact on physical function (gait speed, balance), ADL, and psychological health as well as disease management (Hirvensalo, Rantanen, & Heikkinen, Reference Hirvensalo, Rantanen and Heikkinen2000). A 2015 randomized control trial assessing physical function and hospital readmission rates in older adults with deconditioning undergoing hospital-based rehabilitation found a decrease in readmission rates 30 days post-hospital discharge (Kim et al., Reference Kim, Lee, Han, Lam, Bukowy, Rao and Yoo2015). However, these older adults did not demonstrate significant improvements in ADL as measured by the Katz ADL Index, which may be due to the short duration of rehabilitation (Kim et al., Reference Kim, Lee, Han, Lam, Bukowy, Rao and Yoo2015).
Kortebein (Reference Kortebein2009) conducted a literature review that examined the benefits of a multidisciplinary, traditional rehabilitation program model (subacute and acute rehabilitation wards) for older adults with hospital-acquired deconditioning (HAD) resulting from a prolonged stay. Improvements in function were found, and these older adults were able to successfully transition home. Kortebein suggested that patients should be assigned to their rehabilitation program depending on the amount of rehabilitation the older adult patient is able to withstand per session. An evaluation study by Ottenbacher et al. (Reference Ottenbacher, Smith, Illig, Linn, Ostir and Granger2004) found that while 71 per cent of older adults participating in a traditional rehabilitation program returned to living in the community, 29 per cent were either admitted into institutionalized care or re-admitted to the hospital post-rehabilitation. Thus, it seems that not all older adults are able to benefit from the shorter duration and higher intensity traditional rehabilitation program model to the same extent and may require a different model of care.
Slow Stream Rehabilitation
Older adults with a greater number of co-morbidities and more serious health conditions tend to make smaller functional gains and require longer lengths of hospital stays (Patrick et al., Reference Patrick, Psych, Knoefel, Coe, Gaskowski and Rexroth2001). It is thought that older adults with complex health problems such as multiple co-morbidities, severe stroke, dementia, and frailty may not be able to withstand the typical shorter duration and higher intensity of traditional rehabilitation programs, and may struggle to rehabilitate back to independent living (GTA Rehab Network, 2008). A review assessing the prognosis for functional recovery of older adults in Canadian hospitals found that older adults who are discharged from hospital with new or additional disability in ADL require a longer duration of rehabilitation than current traditional rehabilitation programs (Kortebein, Reference Kortebein2009).
Due to decreased therapeutic gains, the rising number of older adults with complex health problems, and the need to address the problems of traditional rehabilitation for a complex older adult population, some countries have introduced slow stream rehabilitation (SSR) programs into CCC units, stroke rehabilitation units, in-patient rehabilitation units, and nursing homes (South West LHIN, 2009; Sutherland & Trafford Crump, Reference Sutherland and Trafford Crump2013). SSR programs were first introduced in Australia in nursing homes in 1987 as a way of maintaining function for severely deconditioned older adults who resided in nursing homes (O’Neill, McCarthy, & Newton, Reference O’Neill, McCarthy and Newton1987). SSR programs tend to be lower intensity and of longer duration, and to target older adults who have multiple complex health problems and who may not tolerate or benefit from traditional rehabilitation (GTA Rehab Network, 2008). The only literature review completed to date on the topic of SSR is a grey literature scoping review exploring SSR for people with acquired brain injury (ABI) (Piccenna, Knox, & Jacinta, Reference Piccenna, Knox and Jacinta2016). The authors, who found SSR to be beneficial for adults and older adults with ABI, described SSR as being multidisciplinary (based on personally relevant goals and the needs of the individual), outcome driven, and bridging an integrated model of functioning disability and health.
Despite the growing body of research on the benefits of rehabilitation for older adults, we found a large variation in rehabilitation programs that are offered and no clear parameters of who may benefit the most from different models of care. No literature to date has attempted to explore the characteristics of older adults attending SSR programs, SSR program characteristics (e.g., duration [total number of days spent in SSR]; SSR intensity [frequency and amount of time spent in an individual rehabilitation session]; or health professionals involved in SSR), and the benefits of SSR for older adults.
The primary purpose of the scoping review we conducted was to summarize the current body of literature related to SSR for older adults in single-payer health care systems, where “single payer” or “single payer–like” refers to health care funded by the government either through government or quasi-government organizations (World Health Organization, 2018).
Methods
The Canadian Institute of Health Research defines a scoping review as a methodology that aims to explore the breadth of literature on a topic of interest; systematically map the findings; and identify key concepts, theories, gaps, and future direction (Hidalgo Landa, Szabo, Le Brun, Owen, & Fletcher, Reference Hidalgo Landa, Szabo, Le Brun, Owen and Fletcher2011). We used the framework proposed by Arksey and O’Malley, and the suggestions proposed by Levac et al. (Reference Levac, Colquhoun and O’Brien2010), to guide the current scoping review steps and processes (Levac et al., Reference Levac, Colquhoun and O’Brien2010). This framework entails five methodological steps: (a) identify the research question, (b) identify relevant studies, (c) select the studies, (d) chart the data, and (e) collate, summarise, and report the results (Levac et al., Reference Levac, Colquhoun and O’Brien2010).
Step 1: Identify the Research Question
We developed the following research questions with a focus on SSR programs that are available for older adults in single-payer or single payer–like health care systems: What are the characteristics of the older adult patient population (aged 60 years and older) participating in SSR programs? What are the characteristics of SSR programs for older adults with regards to program duration, intensity, setting/location, and clinical practitioners involved? What are the functional, physical, and other outcomes of SSR programs for older adults? To reduce the confounders related to privatized health care systems and to ensure that the results had direct application to the Canadian health care system, we chose to focus on countries with single-payer or single payer–like health care systems.
Step 2: Identify Relevant Studies
The search terms we identified were based upon review of relevant literature and consensus between two authors (MM, SS) (Table 1). We subsequently conducted a three-step search strategy to identify all relevant journal articles and grey literature documents. The first search involved two databases, CINAHL and OVID, in order to identify terms that were synonymous with SSR. Phrases from titles, abstracts, and search terms were then included in the search strategy. Prior to a second search, we consulted with an expert health science librarian for finalization of search terms and search strategy. The second search using all identified search terms and combinations (Table 1) occurred in five primary literature databases (CINAHL, Cochrane, Web of Science, OVID Medline, and OVID Embase), and three grey literature databases (Canadian Public Policy Collection and Global Health, Global Health, and Public Affairs Information Services [PAIS]), in order to cast a wide net and to encompass a variety of settings in which rehabilitation takes place – for example, community, hospital, and nursing homes. The third search we conducted involved reference lists of selected articles that we searched to identify any missing resources. For purposes of searching the databases, all sources of information were potentially eligible in order to capture a broad breadth of primary and grey literature, including policy papers. No date restrictions were applied in order to understand the manifestation and history of SSR (Table 2). Literature sources had to be written in English or published with English translation.
Table 1: Example of search strategy used across all databases

Note. *used in search databases as a wildcard to broaden the search by finding all derivations of the word “age”.
Table 2: Document inclusion and exclusion criteria for screening and full-text phases
Step 3: Select the Studies
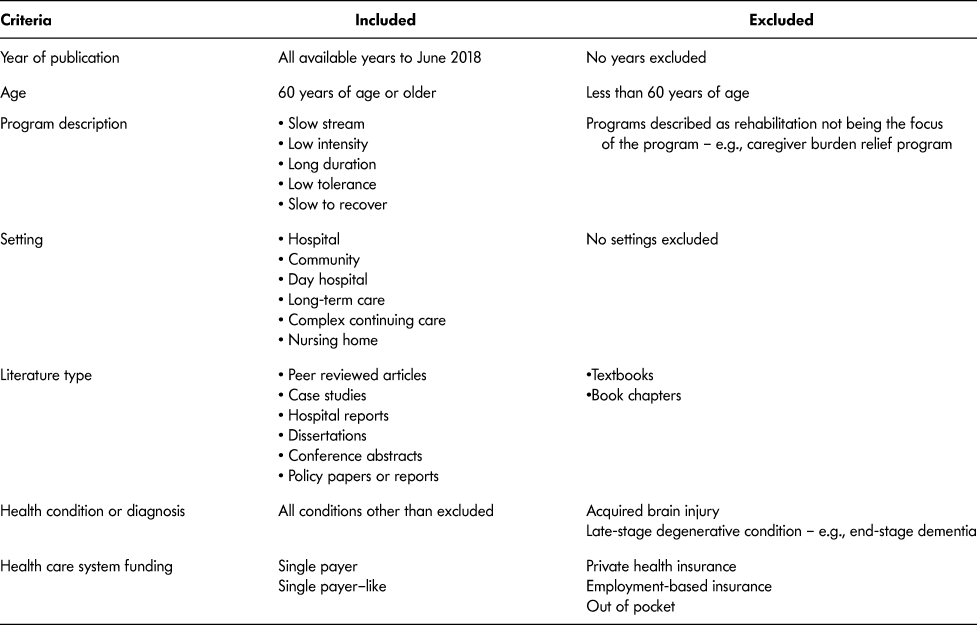
Because the intent of the scoping review was to capture a wide breadth of literature, we used the following inclusion criteria:
(1) Population included were older adult participants aged 60 years and older (World Health Organization [WHO], 2002). We used the WHO definition of older adult, anticipating that literature and documents would originate from different countries.
(2) Any health condition or diagnosis, except ABI or end-stage degenerative disease.
(3) Rehabilitation had to be described as one or more of the following: slow stream, low intensity, long duration, low tolerance, slow to recover. These terms were chosen based upon a review of the literature and the Toronto Rehabilitation Framework (GTA Rehab Network, 2008). Intensity was considered in the context of the amount of rehabilitation time for sessions – for example, amount of time for an individual session and frequency per week, whereas duration was considered as the total number
of days within the SSR program. No cut-off values for either were considered due to the current lack of available operational definitions or empirical values;
(4) All types of rehabilitation settings.
(5) Health care systems similar to that of Canada – for example, single payer or single payer–like. We did not have an a priori list of countries with single-payer health care systems, rather countries as identified in articles and documents were deemed eligible for inclusion through further research of the health care system;
(6) All publication dates to June 2018.
(7) Peer-reviewed papers (quantitative and qualitative methodologies), case studies, conference abstracts, dissertations, hospital reports, policy papers.
To keep the patient population consistent (Mlinac & Feng, Reference Mlinac and Feng2016), we did not include papers or documents that described SSR (a) years after initial onset of health condition or diagnosis; (b) for end-stage degenerative conditions, as the focus would be palliative care; (c) as programs whose primary purpose was caregiver relief. We did not include ABI, as Piccenna et al. (Reference Piccenna, Knox and Jacinta2016) conducted a scoping review related to this diagnosis. Last, we also did not include textbooks or book chapters. Table 2 shows the complete list of document inclusion and exclusion criteria.
Titles and abstracts were imported into Mendeley Version 1.19.2 (2008–2018 Mendeley Ltd.), and duplicates were automatically removed by the Mendeley program. Titles and abstracts were then independently reviewed by two author reviewers (MM, SS) based upon the inclusion and exclusion criteria. Disagreements were resolved via discussion with a third author reviewer (VBDH). Full-text data extraction was independently undertaken. A Kappa value was calculated using SPSS version 24. We did not determine the Kappa value a priori, but we were looking for substantial agreement. It is suggested that Kappa results be interpreted as following: values ≤ 0 as no agreement, 0.01–0.20 as slight agreement, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement (McHugh, Reference McHugh2012), thus anything above 0.61 would have been deemed acceptable.
Steps 4 and 5: Chart, Collate, Summarise, and Report the Results
To document information from the included published articles and grey literature, an Excel spreadsheet was created and securely hosted online, so that all research team members had access. We extracted details regarding publication year, country of publication, methodology, objective(s), sample size, participant characteristics (e.g., age, sex, number of co-morbidities), program description, length of stay, outcome measures used (e.g., physical outcomes, ADL measures) and discharge destination.
According to Levac et al. (Reference Levac, Colquhoun and O’Brien2010), part of collating, summarizing, and reporting of the results is to map the findings and produce a numerical analysis of the extent and nature of studies using tables and charts. Accordingly, we included tables and reported the range of means. To answer the first research question, we reported the range of means for the following across the literature documents: age, number of co-morbidities, sex percentage, diagnosis, or reason for rehabilitation. To answer the second research question, we reported the range of means across the literature documents for total SSR program duration (length of stay, or LOS), intensity – frequency (number of individual sessions per week), and amount of time spent in an individual session. In addition, we extracted the composition of the SSR team. To address the benefits of SSR programs for older adults, we also extracted (e.g., means reported) the outcome measures used and results.
Results
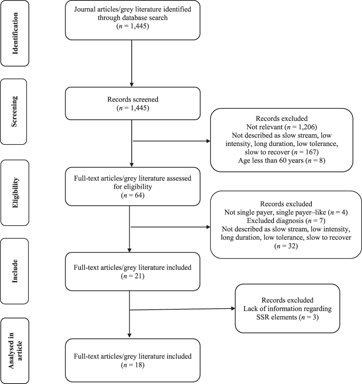
A total of 1,445 literature documents were screened by two reviewers (MM, SS) with a Cohen Kappa value of 0.78, (CI = 0.73, 0.83), which is indicative of substantial agreement. Sixty-four articles and documents remained after assessment for eligibility. Reasons for exclusion at this point were as follows: the program was not an SSR program (n = 32); government did not fund the program – the older adult individuals had to pay out of pocket for rehabilitation; all four programs were conducted in the United States whose health care system is not single payer (n = 4); and age, health condition, or diagnosis did not meet the inclusion criteria (n = 7; for example, ABI in young adults; diagnosis of stroke 10 years ago; Down syndrome) (Figure 1).

Figure 1: Flow diagram of process of identification and selection of relevant studies and documents, including the number of studies screened and excluded at each stage
After initial and full text review, we included 21 primary articles and grey literature documents: 11 peer-reviewed articles, five conference abstracts, and five report documents. Three documents (Englund, Reference Englund1987; Raymond, Winter, & Holland, Reference Raymond, Winter and Holland2015; Wilson & Ballentyne, Reference Wilson and Ballentyne2017) did not describe the SSR program or outcomes of the program, and therefore we later excluded them in the data extraction phase: (a) one of the three excluded documents was a measurement study aimed at validating an activity monitor in a hospital-based SSR setting (Raymond, Winter, & Holland, Reference Raymond, Winter and Holland2015; peer-reviewed); (b) one of three excluded was a critique of the methodology used in O’Neill et al.’s Reference O’Neill, McCarthy and Newton1987 article and a response to the critique in 1987 (Englund, Reference Englund1987; peer-reviewed); and (c) the last excluded document was a description of the role of occupational therapists in SSR (Wilson & Ballentyne, Reference Wilson and Ballentyne2017; conference abstract). Ultimately, 18 included literature documents remained – nine peer-reviewed articles, four conference abstracts, and five report documents.
The final 18 literature documents were published in four different countries: Australia (O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker, Hill, Cobden, Davidson, & McBurney, Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al., Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995); Canada (ALC Expert Panel, 2006; Berall, Naglie, Katz, Chang, & Leung, Reference Berall, Naglie, Katz, Chang and Leung2013; GTA Rehab Network, 2008; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Kubilius, Rose, Pettit, & St. Amant, Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Ontario Hospital Association, 2006; Ontario Stroke Network, 2013; South West LHIN, 2009; Teasell, Foley, Bhogal, Chakravertty, & Bluvol, Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011); Singapore (Chong, Empensando, Ding, & Tan, Reference Chong, Empensando, Ding and Tan2012; Zhang, Ang, & Kwek, Reference Zhang, Ang and Kwek2015); and the Netherlands (Spruit-van Eijk, Zuidema, Buijck, Koopmans, & Geurts, Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012) (Table 3).
Table 3: Characteristics of literature documents included in scoping review
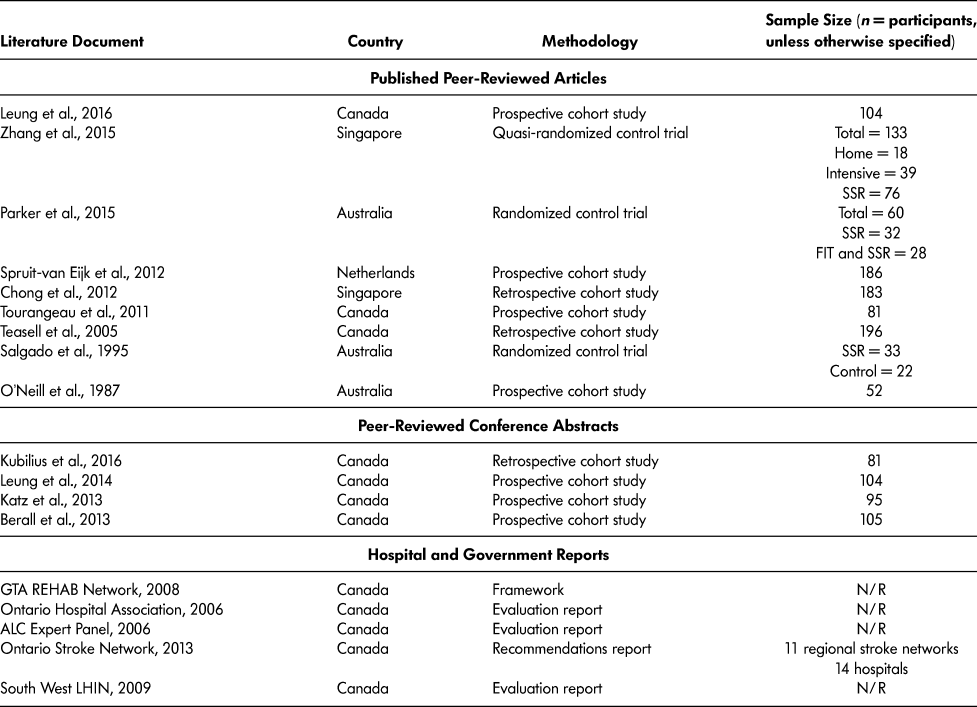
Note. FIT = functional individual training; N/R = not reported; SSR = slow stream rehabilitation.
SSR research originated in Australia in 1987 and publications continued until 1995. From 1995 to 2005, there were no SSR-related publications. In 2005, the first Canadian SSR paper was published, describing SSR in the hospital setting for older adults with severe stroke who could not withstand traditional hospital rehabilitation (Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005). Since 2005, there have been 12 Canadian SSR-related documents published (Table 3). Of the 13 peer-reviewed articles and conference abstract included, 10 (76.9%) were cohort studies – three retrospective cohort studies (Chong et al., Reference Chong, Empensando, Ding and Tan2012; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) and seven prospective cohort studies (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). There were three randomized control trials (RCT) conducted to compare SSR to different models of care (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al., Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; Zhang et al., Reference Zhang, Ang and Kwek2015). Refer to Table 3 for the list of literature documented and their methodology.
Within the five report documents, there was one report describing a hospital framework (GTA Rehab Network, 2008), three hospital evaluation reports (ALC Expert Panel, 2006; Ontario Hospital Association, 2006; South West LHIN, 2009), and one stroke rehabilitation recommendation report (Ontario Stroke Network, 2013). The geriatric rehabilitation framework report published by the Greater Toronto Area (GTA) Rehab Network discussed the differing types of geriatric in-patient rehabilitation units available to older adult patients and gave guidelines as to when an SSR program should be used and what an SSR program should entail (GTA Rehab Network, 2008). Two of the three hospital evaluation reports assessed hospital-based rehabilitation in CCC units in Ontario (South West LHIN, 2009; Ontario Hospital Association, 2006) and reported lack of clarity, lack of information, and lack of resources available for health care practitioners when making rehabilitation decisions regarding CCC rehabilitation for older adult patients. The report by the LHIN concluded that many CCC programs and rehabilitation programs were not appropriately utilized and that transition and referral processes need to be enhanced (South West LHIN, 2009). The last hospital report, written by an expert panel, was ALC focused with the aim of assessing levels of care and flow of care into in-patient SSR units (ALC Expert Panel, 2006). The ALC panel reported that patient flow to SSR occurred following specialized rehabilitation when an older adult was considered stable but unable to return to community living (ALC Expert Panel, 2006).
Finally, the report conducted by the Ontario Stroke Network compared the use of SSR in CCC hospital units to an active stroke rehabilitation unit for patients with stroke. The Ontario Stroke Network found that older adult individuals with severe stroke who were admitted to an active stroke rehabilitation program had a shorter length of stay and similar functional outcomes. The Ontario Stroke Network (2013) recommended that older adult patients, who could potentially withstand active stroke rehabilitation, would be better served by admission to active stroke in-patient rehabilitation than by an SSR program in CCC.
Characteristics of SSR Program for Older Adults
Of all 18 reported literature documents, 15 described staff available in SSR programs (ALC Expert Panel, 2006; Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; GTA Rehab Network, 2008; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Ontario Stroke Network, 2013; Ontario Hospital Association, 2006; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al., Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; South West LHIN, 2009; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Zhang et al., Reference Zhang, Ang and Kwek2015) (Table 4). All 15 described SSR programs as multidisciplinary, and included a physiotherapist, occupational therapist, and nurse practitioner or physician as part of the rehabilitation team. Other health care professionals included on SSR teams were as follows: physiotherapy assistant in six of the 15 programs, an occupational therapy assistant in four of the 15 programs, social worker in five of the 15 programs, speech language pathologist in eight of the 15 programs, dietician in seven of the 15 programs, and recreational therapist in three of the 15 programs.
Table 4: Characteristics of slow stream rehabilitation programs
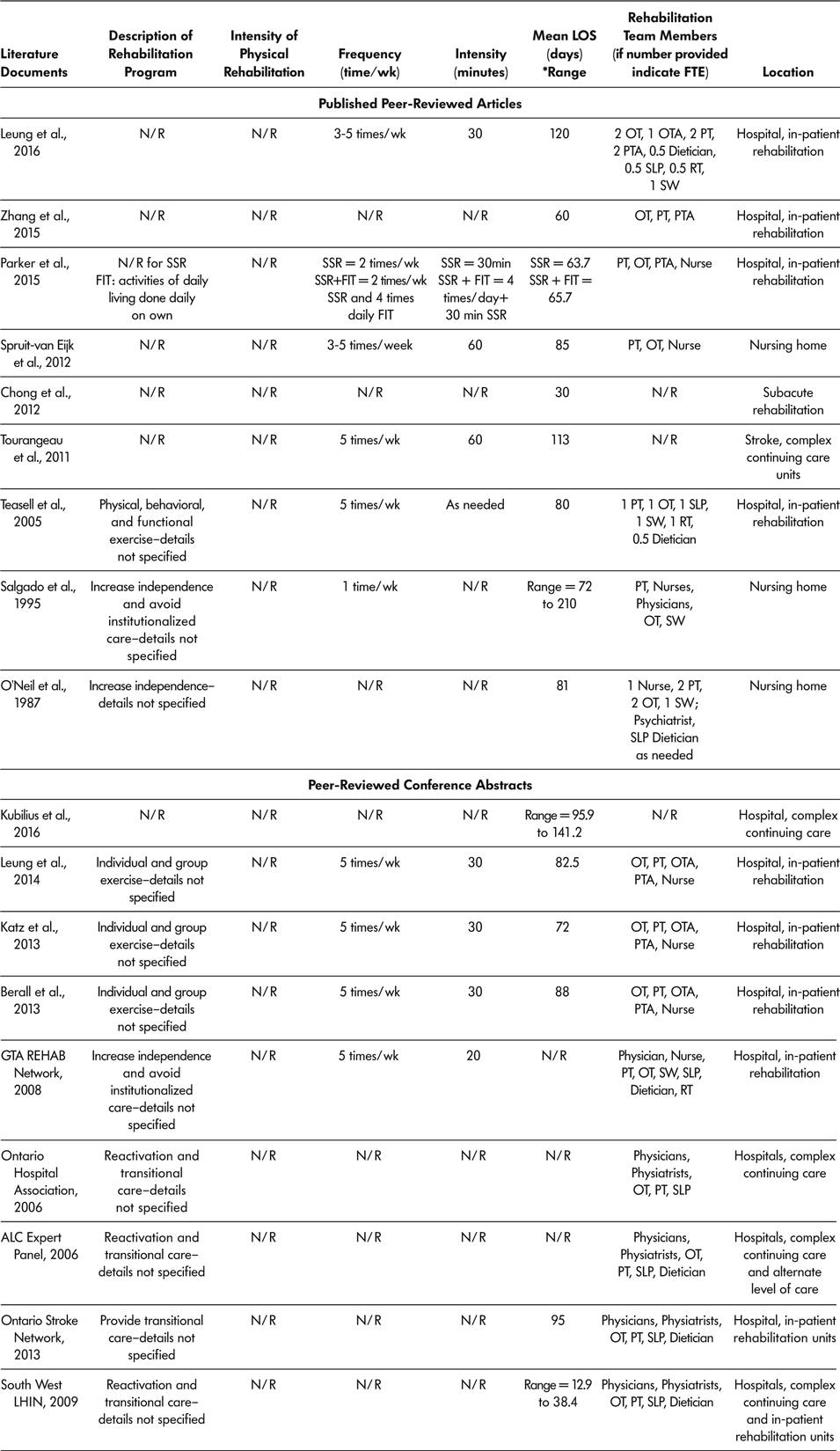
Note. ALC = alternate level of care; FIT = functional individual training; FTE = full time equivalent; LIHN = Local Health Integrated Network; LOS = length of stay; N/R = not reported; OT = occupational therapist; OTA = occupational therapist assistant; PT = physiotherapist; PTA = physiotherapist assistant; RT = recreational therapist; SLP = speech language pathologist; SW = social workers; SSR = slow stream rehabilitation; wk = week.
Total SSR program duration (LOS) was recorded for 15 of the 18 (83%) literature documents, with a range across literature documents of 30 days to 141.2 days (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Chong et al., Reference Chong, Empensando, Ding and Tan2012; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Ontario Stroke Network, 2013; Salgado et al., Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; South West LHIN, 2009; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011; Zhang et al., Reference Zhang, Ang and Kwek2015). Only 10 of 18 (55%) included documents described the SSR session intensity (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; GTA Rehab Network, 2008; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al.,Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). The number of rehabilitation sessions attended by participants per week varied from once a week (Salgado et al.,Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995) to five times per week (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; GTA Rehab Network, 2008; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011).
The amount of time of each rehabilitation session ranged from 20 minutes (GTA Rehab Network, 2008) to 60 minutes (Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). For all 18 included documents, SSR programs were offered as in-patient programs, meaning the older adult stayed overnight at the rehabilitation location. Nine (50%) of the 18 SSR programs took place in in-patient hospital rehabilitation wards (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; GTA Rehab Network, 2008; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Zhang et al., Reference Zhang, Ang and Kwek2015; Ontario Stroke Network, 2013); five (27.8%) in CCC units (ALC Expert Panel, 2006; Ontario Hospital Association, 2006; South West LHIN, 2009; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011); three (16.7%) in nursing homes (O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Salgado et al., Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012); and one (5.5%) in a subacute rehabilitation (Chong et al., Reference Chong, Empensando, Ding and Tan2012). Table 4 lists characteristics of SSR programs.
Characteristics of Older Adults Participating in SSR Programs
Age was reported in 16 of the 18 literature documents, with youngest reported mean age being 72 years (Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) and oldest reported mean age being 82 years (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Salgado et al., Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995). Eleven (61.1%) of 18 included documents provided information regarding sex distribution of SSR participants (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Chong et al., Reference Chong, Empensando, Ding and Tan2012; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011; Zhang et al., Reference Zhang, Ang and Kwek2015). The percentage of female participants ranged from 47 per cent (Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) to 81 per cent (Zhang et al., Reference Zhang, Ang and Kwek2015). Across all 18 included literature documents, six (Chong et al., Reference Chong, Empensando, Ding and Tan2012; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; South West LHIN, 2009; Zhang et al., Reference Zhang, Ang and Kwek2015) reported patients’ co-morbidities, with the lowest mean number of co-morbidities being 1.7 (Chong et al., Reference Chong, Empensando, Ding and Tan2012) and the highest mean being 7.3 (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015). Primary diagnosis was reported in all 18 literature documents. Multiple primary diagnoses were reported with the most common primary diagnoses of older adult SSR participants being stroke, deconditioning, orthopaedic conditions, chronic complex health conditions, surgery, cognitive impairments, frailty, and falls. Secondary diagnosis was reported in nine of the 18 literature documents and included multiple chronic complex conditions, cognitive impairment, and frailty. See Table 5 for demographics and health history of older adults attending SSR programs.
Table 5: Demographics and health history of older adult population attending slow stream rehabilitation programs according to demographics provided in included literature documents
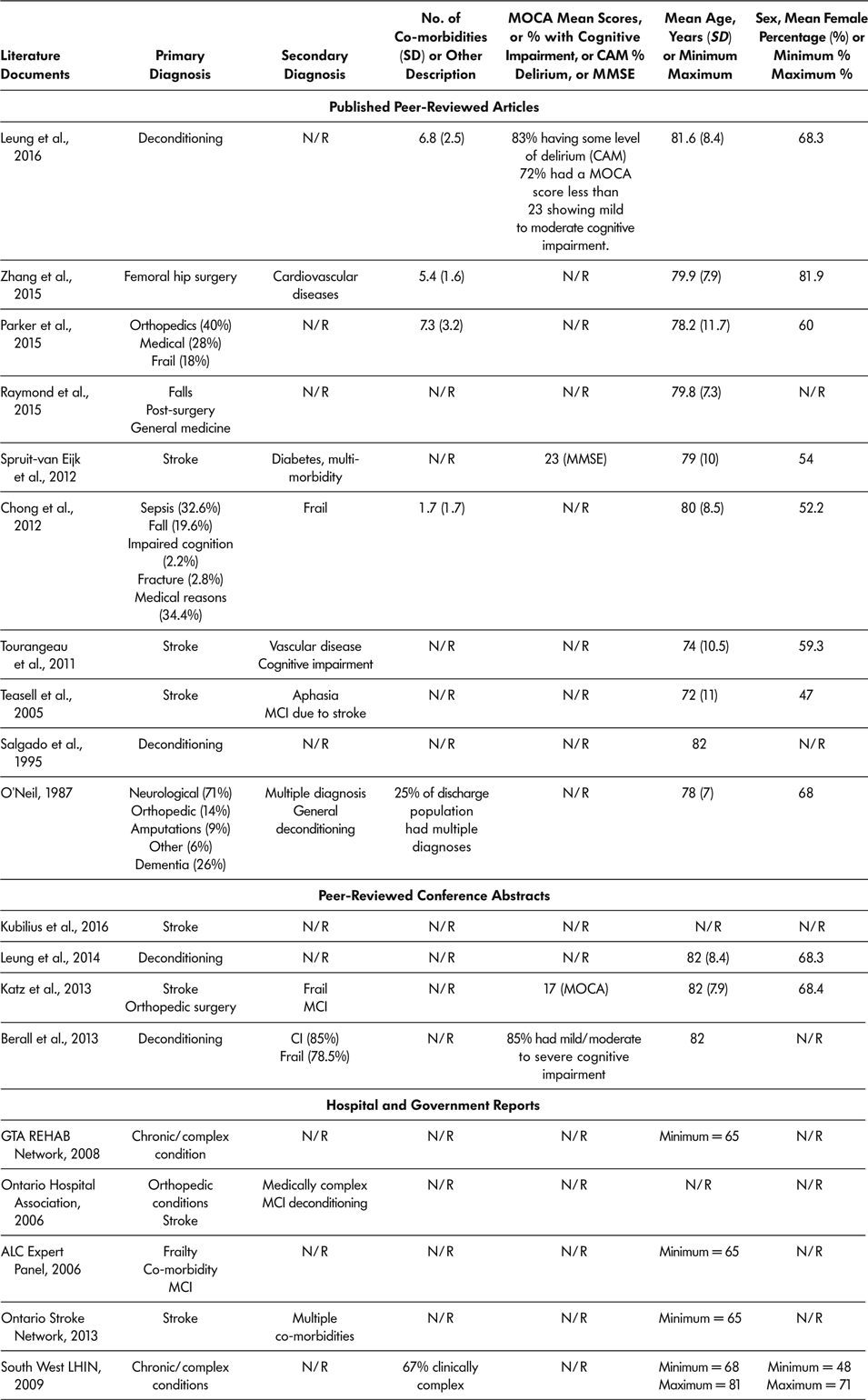
Note. ALC = alternate level of care; CAM = confusion assessment method; CI = cognitive impairment; LIHN = Local Health Integrated Network; MC = mild cognitive impairment; MMSE = Mini Mental State Exam; MOCA = Montreal Cognitive Assessment; N/R = not reported in the literature document; SD = standard deviation; SSR = slow stream rehabilitation.
Cognitive Ability
Four literature documents used a measure of cognitive ability at baseline, and these documents reported that most of the older adult participants had some level of cognitive impairment or delirium (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012). Leung et al. (Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016) reported that 72 per cent of participants had some cognitive impairment, and 83 per cent had some level of delirium (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016). Similarly, Berall et al. reported that 85 per cent of participants had mild to moderate cognitive impairment on admission (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013). Spruit-van Eijk et al. (Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012) reported a mean Mini-Mental State Exam (MMSE) score of 23, indicative of mild cognitive impairment.
Outcome Measures Used in Slow Stream Rehabilitation for Older Adult Participants
For a summary of included documents, outcome measures used, and reported findings, see Tables 6a and 6b. The majority of documents (13 of the 18, 72.2%) used outcome measures to describe or assess the SSR program (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Chong et al., Reference Chong, Empensando, Ding and Tan2012; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al.,Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011; Zhang et al., Reference Zhang, Ang and Kwek2015). Over three quarters (76.9%, 10 of 13) of the literature documents that used outcome measures used a measure of ADL or function to assess change from baseline to discharge (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). The most commonly used measure was the Functional Independence Measure (60%, 6 of 10) (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005), but others included the ADL hierarchy (Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011), de Morton Mobility Index (DEMMI) (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015), Barthel Index (BI) (Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012), and one tool created by the authors to measure dependency level for completion of ADL (O’Neill et al., Reference O’Neill, McCarthy and Newton1987).
Table 6a: Summary of published peer-reviewed articles and conference abstracts included in the scoping review
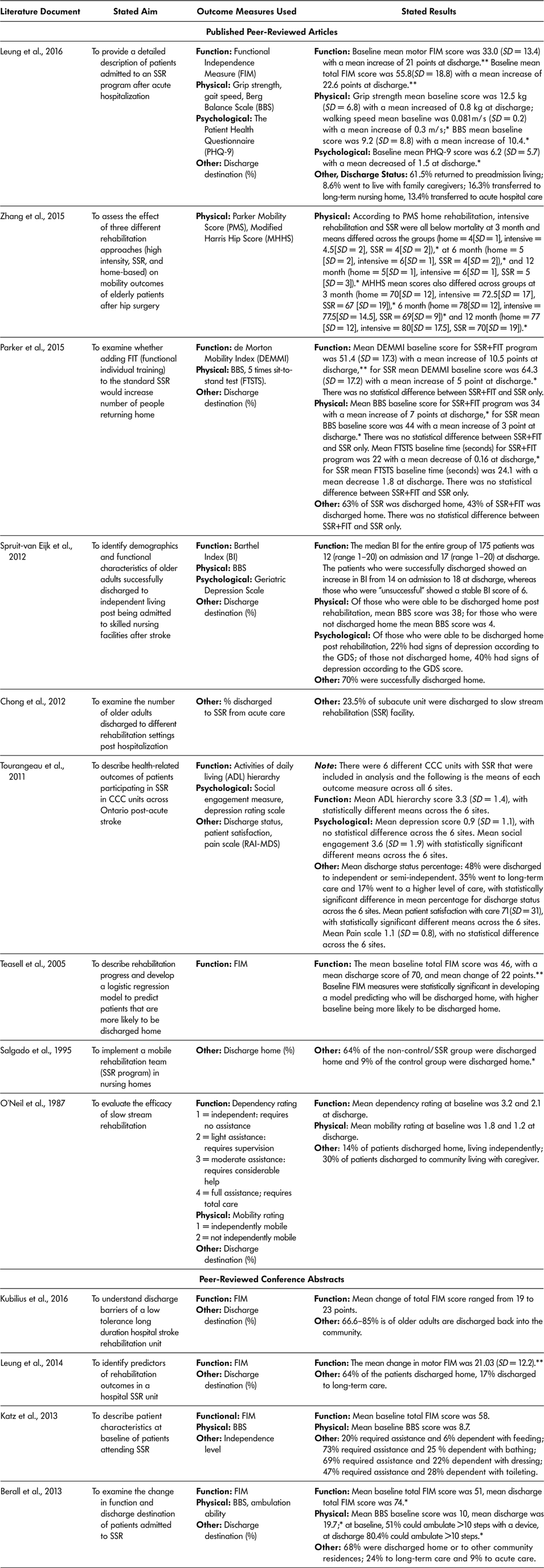
Note. * statistically significant result according to study; ** clinically significant result according to study. BBS = Berg Balance Scale; FIM = Functional Independence Measure; SD = standard deviation; SSR = slow stream rehabilitation.
Table 6b: Summary of report documents included in the scoping review

Note. ALC = alternate level of care; CCC = complex continuing care; LHIN = Local Health Integrated Network; LOS = length of stay; SSR = slow stream rehabilitation.
Researchers used physical outcome measures to assess change from SSR admission to discharge in seven of 13 (53.8%) literature documents (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Zhang et al., Reference Zhang, Ang and Kwek2015). The most often-used measure was the Berg Balance Scale (71.4%), accounting for five of the seven literature documents examining physical outcomes (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012). Other physical outcome measures applied included the Parker Mobility Score (Zhang et al., Reference Zhang, Ang and Kwek2015), Modified Harris Hip Score (Zhang et al., Reference Zhang, Ang and Kwek2015), five times sit-to-stand test (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015), ambulation ability or speed (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016), grip strength (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016), and a researcher-designed mobility score (O’Neill et al., Reference O’Neill, McCarthy and Newton1987).
Only three of the 13 (23.1%) included literature documents included psychological or other outcome measures including (a) the patient Health Questionnaire (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016), which includes questions about mental and emotional status, such as feelings of depression; (b) Geriatric Depression Scale (GDS) (Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012), and (c) a measure of social engagement (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016)
Outcomes of Slow Stream Rehabilitation for Older Adults
Changes in Function and Activities of Daily Living
Baseline mean total FIM scores across the six literature documents (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) ranged from 46 (Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) to 55.8 (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016). Discharge mean FIM scores across the six literature documents (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) ranged from 70 (Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005) to 78 (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016), with all the changes from baseline to discharge being reported as both clinically and statistically significant. Other ADL measures used such as the Barthel Index (Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012), the ADL hierarchy scale (Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011), the de Morton Mobility Index (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015), and dependency rating (O’Neill et al., Reference O’Neill, McCarthy and Newton1987) all showed improvements from baseline to discharge.
Changes in Physical Outcomes
The five literature documents (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Spruit-Van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015) that used the Berg Balance Score (BBS) as a physical outcome measure reported an increase in the BBS from baseline to post-SSR. The mean change in BBS score from baseline to discharge ranged, in points, from three (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015) to 10 (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016). Older adult participants with the greatest increase in BBS score completed SSR in an in-patient hospital rehabilitation unit and had the lowest mean BBS baseline scores: 9.2 (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016). Older adult participants showing the smallest mean change in BBS had a higher baseline mean score (44), greater functional ability, and were participating in an RCT study wherein SSR as standard care was compared to SSR plus additional functional exercises (Parker et al. Reference Parker, Hill, Cobden, Davidson and McBurney2015) – SSR-only participants scored a 3-point mean increase in BBS whereas those in the SSR plus additional functional exercise had a 7-point mean increase. All other literature documents that applied physical outcome measures (walking speed, grip strength, or mobility measures) found statistically significant increases in scores from baseline to discharge of SSR, but none reported whether a clinically significant change was achieved (Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Zhang et al., Reference Zhang, Ang and Kwek2015).
Of all included literature documents assessing SSR programs, only one literature document examined the long-term benefits. Zhang et al. (Reference Zhang, Ang and Kwek2015) conducted a quasi-RCT comparing home rehabilitation, intensive rehabilitation, and SSR for older adults at 3, 6, and 12 months post-femoral fracture. Zhang et al. (Reference Zhang, Ang and Kwek2015) found that there were no differences in walking ability (Parker Mobility scores) between home care and SSR at any time point, but intensive rehabilitation was effective in improving walking ability (Parker Mobility scores) and function (Mod Harris Hip score) at all-time points.
Changes in Psychological Measures
Of the three literature documents that assessed changes in emotional or psychological states, two found a decrease in depression scores from baseline to discharge using a Depression Rating Scale (Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011) and the Patient Health Questionnaire (Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016). Spruit-van Eijk (Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012) found that those discharged home were less likely to have depression (23% with depression as measured by the Geriatric Depression Scale, GDS) in comparison to those discharged to long-term care (40% with depression as measured by the GDS).
Discharge Destination
Ten (55.6%) of the 18 literature documents included discharge destination (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Chong et al., Reference Chong, Empensando, Ding and Tan2012; Kubilius et al., Reference Kubilius, Rose, Pettit and St. Amant2016; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al.,Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). The reported range across literature documents of mean percentage of older adult participants who were discharged back into the community after SSR were 44 per cent to 70 per cent. The literature documents with the highest discharge rates to home described SSR programs based in in-patient units with an average LOS of 85–88 days (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012). Older adult participants had an average age range of 79–82 years and also had cognitive impairment. The lowest discharge rates to home were from nursing home–based SSR programs, with an average LOS of 81 days. Older adults had an average age of 78 years, and 81 per cent were considered to have neurological deficits and an average dependency rate of 2.1 out of 4, meaning that they required light assistance with ADL (O’Neill et al., Reference O’Neill, McCarthy and Newton1987).
Discussion
The aim of this scoping review was to develop a greater understanding of the available literature on SSR programs, within single-payer or single payer–like health care systems. Through report documents, empirical literature, and research abstracts, this scoping review illustrates the similarities between SSR programs, highlights the differences and areas for improvement, discusses the benefits for older adults participating in SSR, identifies the role of SSR programs in Canadian health care, and proposes a need for continued research.
Slow Stream Rehabilitation Programs
Similarities across Current SSR Programs
We can surmise from the included literature documents that SSR programs are typically not disease- or health condition-specific, but instead target community-living older adult patients who may be struggling with independent living, have HAD, complex health problems, or cannot be discharged home even after participating in a condition-specific rehabilitation program. SSR programs are offered as in-patient rehabilitation programs or are integrated into hospitals (ALC, CCC, hospital in-patient rehabilitation) and nursing homes, with the goal of discharging the older adult back into the community and avoiding institutionalized care. SSR programs are multidisciplinary, encompassing a physiotherapist (PT), occupational therapist (OT), and physician or nursing staff, and in some cases include other health professionals for some SSR models – for instance, PT or OT assistants, dieticians, speech language pathologists (SLP), and recreational therapists. The most common rehabilitation set-up for SSR programs is five times a week for 30 minutes a day with a two- to three-month length of stay (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; GTA Rehab Network, 2008; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; Teasell et al., Reference Teasell, Foley, Bhogal, Chakravertty and Bluvol2005; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). SSR programs that focused on functional exercises and had dieticians, SLP, and recreational therapists in addition to PT, OT, and a physician or nurse on the team tended to show the greatest benefits.
Differences and Shortcomings of Current SSR Programs
The major differences we found in the SSR programs that were included in this scoping review relate to (a) the frequency and duration of the individual sessions, (b) the total length of the program, and (c) the various SSR program locations: for example, in-patient acute ward, CCC units, and nursing homes. The available resources and demands of particular SSR programs included may play a role in how the program is structured in terms of length of stay, extent of daily rehabilitation received, and the composition of the rehabilitation team. SSR programs that took place in nursing home or stroke units tended to have the longest LOS, as the patients presented with greater disability according to baseline scores and could not as readily be discharged home (Salgado et al.,Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995; Spruit-van Eijk et al., Reference Spruit-van Eijk, Zuidema, Buijck, Koopmans and Geurts2012; Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011). In comparison, programs that took place in subacute care units had the shortest LOS (Chong et al., Reference Chong, Empensando, Ding and Tan2012). The duration of SSR should be dependent upon the older adults’ progression and meeting of goals. Thus, location for SSR programs should be one that can offer longer durations and fewer pressures for health care providers to discharge the patient as quickly as possible.
The major limitation of the included documents was the lack of specific information regarding the SSR program as to whether it comprised (for example) specific exercises (type or intensity); specific interventions such as PT, OT, SLP, nursing or recreation therapy interventions; the referral process; goals specific to the older adult patient and knowledge regarding SSR programs offered for older adults; the discharge process; and so on. None of the 18 included literature documents included specific information regarding the details of a rehabilitation program, which poses barriers for (a) implementing it in community programs or hospitals that wish to introduce SSR programs into their organizations; (b) ensuring fidelity of the interventions; and (c) comparing the benefits of SSR programs to other programs, such as home rehabilitation or traditional rehabilitation. The lack of information regarding the referral process may lead to suboptimal patient flow and health care provider confusion. The ALC Expert Panel (2006), Ontario Stroke Network (2013), and South West LHIN (2009) documents all indicated that hospitals need to increase education about available rehabilitation programs and their use, develop a standard definition for the various components within the continuum of care, and identify where different rehabilitation programs fit within the continuum of health care.
In the absence of these strategies, issues related to improper program implementation and lack of appropriate pathways for the older adult patient could lead to older adults with complex health needs being more likely to be discharged into institutionalized care (ALC Expert Panel, 2006; Ontario Stroke Network, 2013; South West LHIN, 2009), rather than benefitting from a longer-duration, low-intensity program. Last, none of the 18 articles or documents reported the older adult patients’ personal goals. This could be an issue because older adults may meet the program goals or goals set by the health care professional but may be discharged home without having their own goals met. For example, if an older adult’s specific goal is to return to attending a weekly community-based social gathering, she may experience isolation, depression, and decreased quality of life if she did not achieve her personal goal even though her scores on functional measures improved prior to discharge home. Furthermore, research has shown that when patients are involved with setting their own goals and set goals they perceive as important, they are more likely to be more independent (Reuben & Tinetti, Reference Reuben and Tinetti2012; Schulman-Green, Naik, Bradley, McCorkle, & Bogardus, Reference Schulman-Green, Naik, Bradley, McCorkle and Bogardus2006).
SSR Programs and Older Adults
Similarities of Older Adults Participating in SSR Programs
According to our findings, SSR programs most often serve older adults who are in their 70s and 80s, have the lower baseline physical function scores compared to age-normative values (Heinemann, Linacre, Wright, Hamilton, & Granger, Reference Heinemann, Linacre, Wright, Hamilton and Granger1993; Long et al., Reference Long, Sacco, Coombes, Copes, Bullock and Melville1994), have multiple co-morbidities, some level of cognitive impairment, and have HAD (Berall et al., Reference Berall, Naglie, Katz, Chang and Leung2013; Chong et al., Reference Chong, Empensando, Ding and Tan2012; Katz et al., Reference Katz, Naglie, Berall, Karuza, Chan and Leung2013; Leung et al., Reference Leung, Katz, Karuza, Chan, Berall, Fallah and Naglie2014; Leung et al., Reference Leung, Katz, Karuza, Arling, Chan, Berall and Naglie2016; O’Neill et al., Reference O’Neill, McCarthy and Newton1987; Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015; Salgado et al.,Reference Salgado, Ehrlich, Banks, Browne, Buckman and Burraston1995). Essentially, SSR programs have demonstrated benefits for older adults who would typically be considered as having low rehabilitative potential by health care professionals (Burton, Horne, Woodward-Nutt, Bowen, & Tyrrell, Reference Burton, Horne, Woodward-Nutt, Bowen and Tyrrell2015; GTA Rehab Network, 2008; Kortebein, Reference Kortebein2009; Patrick et al., Reference Patrick, Psych, Knoefel, Coe, Gaskowski and Rexroth2001).
We found that 44 per cent to 70 per cent of older adult patients attending SSR programs returned back to independent living in the community. Approximately 75 per cent of older adults’ experience HAD, with HAD being more common in older adults with multi-morbidity, cognitive decline, and low physical function (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003). Rehabilitation programs that target older adults with HAD have been shown to improve long-term survival and function, with most programs being offered in a sub-acute in-patient rehabilitation (low intensity, long duration) setting where the goal is to maximize functional recovery (Kortebein, Reference Kortebein2009).
In SSR programs that reported on a specialized rehabilitation population (stroke and post-femoral surgery), the subpopulation that benefited the most from SSR involved older adults with multiple co-morbidities and low physical function (Tourangeau et al., Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011; Zhang et al., Reference Zhang, Ang and Kwek2015). The findings of this scoping review align with the findings of systematic reviews of rehabilitation post-femoral surgery (Beaupre et al., Reference Beaupre, Binder, Cameron, Jones, Orwig, Sherrington and Magaziner2013; McGilton et al., Reference McGilton, Davis, Mahomed, Flannery, Jaglal, Cott and Rochon2012), wherein older adults with complex health problems, low discharge probability, and cognitive impairment were found to benefit from longer duration, low intensity rehabilitation. Similar trends were found in the stroke literature. Tourangeau et al. (Reference Tourangeau, Squires, Wodchis, McGilton, Teare and Widger2011) found that older adults with severe stroke admitted to CCC were more likely to have mild cognitive impairment, depression, require assistance with ADL, and were considered to have low rehabilitative potential; however, they were also more likely to make significant physical gains during SSR. What remains unknown is the longer-term benefits of SSR in terms of physical improvements and the ability to remain at home.
Differences in Benefits of Older Adults Participating in SSR Programs
Our scoping review found that not all older adults benefit from SSR over traditional rehabilitation (GTA Rehab Network, 2008; Ontario Stroke Network, 2013; Zhang et al., Reference Zhang, Ang and Kwek2015). Older adults who are considered healthier, have a low number of co-morbidities, little cognitive decline, are fairly independent in ADL, and require only specialized rehabilitation may benefit more from more traditional rehabilitation programs. A quasi-randomized trial comparing SSR to intensive specialized rehabilitation for older adults post-hemiarthroplasty found that the short, intensive rehabilitation program was more beneficial than SSR when the older adult participant had fewer co-morbidities, lower mortality scores, and were younger (Zhang et al., Reference Zhang, Ang and Kwek2015). Consequently, not all older adults may benefit equally from SSR and there may be a sub-group of older adults who can withstand and can benefit more from traditional rehabilitation programs.
Similar conclusions can be made regarding SSR for older adults’ post-stroke. Older adults who attended a specialized stroke rehabilitation program were more likely to be readmitted to rehabilitation if they were older, female, unable to complete ADL, and had other complex health issues (Canadian Institute for Health Information, 2009). One of the reports included in this scoping review, an evaluation and recommendations report by the Ontario Stroke Network, indicated that patients with severe stroke who were originally thought to benefit more from rehabilitation in an SSR program actually had better outcomes in more intensive rehabilitation programs, but arguably this meant only those individuals who have the ability to readily be discharged home (Ontario Stroke Network, 2013).
Integrating SSR Programs into Canada’s Current Health Care System
As previously mentioned, all SSR programs included were offered within in-patient settings, yet that may not be Canada’s best option. Housing SSR programs within hospitals places a burden on the health care system and decreases hospital resources. Housing these programs in nursing homes and assisted living facilities may result in increased wait times for patients unable to live independently, or who are waiting for assisted living or long-term care. A more economical model may be to house SSR programs in the community. Bean, Vora, and Frontera, (Reference Bean, Vora and Frontera2004) and Tuntland, Aaslund, Espehaug, Førland, and Kjeken (Reference Tuntland, Aaslund, Espehaug, Førland and Kjeken2015) found that community programs were effective in decreasing mortality, enhancing physiological capacity, increasing overall function, increasing overall health-related quality of life, and preserving the older adult’s ability to live independently. Two reviews conducted a cost-effective analysis of community programs across Australia and the United States and found that programs housed in the community are 20 per cent more cost-effective than in-patient rehabilitation programs (Brown et al., Reference Brown, Forster, Young, Crocker, Benham and Langhorne2015; Kjerstad & Tuntland, Reference Kjerstad and Tuntland2016).
With the growing number of older adults with multiple co-morbidities and complex health problems living in the community (Canadian Medical Association, 2016), the demand for effective rehabilitation models, including SSR models, will only increase. In order to address the burden this will place on hospitals and nursing homes, Canada’s health care system should develop more initiatives focused on community-based rehabilitation that includes physical activity, chronic disease management, and support for older adults to remain in the community post-hospital discharge. Implementation of these programs has great potential to support healthy aging and “aging-in-place” post-hospitalization, as well as the potential to decrease the need for the number of long-term care beds and assisted-living wait times, in addition to the use of ALC and hospital re-admissions.
Future Direction
From this scoping review, we have begun to understand which older adults benefit from SSR programs, where SSR programs are currently housed and, in broad terms, what they encompass. However, there still remain many unanswered questions. More studies and focused program evaluations need to be conducted in order to further understand, better define, and optimize SSR program design. Future studies should assess specifics of program design – for example, the optimal amount of rehabilitation time, optimal length of stay for rehabilitation-related gains, details regarding individual rehabilitation sessions, and specific interventions in order to produce best-practice guidelines for SSR programs. Very few studies to date have compared SSR to other rehabilitation models. In our current search, only one of the three randomized control trials assessed the benefits of adding additional non-supervised exercises to their current SSR programs (Parker et al., Reference Parker, Hill, Cobden, Davidson and McBurney2015). Parker et al. (Reference Parker, Hill, Cobden, Davidson and McBurney2015) did find some improvement in physical function, but the improvements were not statistically significant; this finding may be a result of not having a method to measure adherence in the intervention group. More RCTs need to be conducted in order to assess whether SSR programs have an equivalent or greater effect on increasing functional independence for older adults with complex needs following prolonged hospital compared to more intensive rehabilitation, standard hospital rehabilitation, or home care.
Furthermore, no research has assessed patient-specific goals for SSR programs and how those program goals may compare to the types of patient specific goals being set in traditional rehabilitation programs. There is also no research related to the long-term benefits of SSR programs. Most studies and grey literature documents examined whether older adults were discharged home, or to long-term care or assisted-living facilities post-rehabilitation; however, there was no longer-term follow-up with these older adults. Future research should assess the benefits of SSR programs via long-term follow up – for instance, three months, six months, and one-year post discharge.
Finally, in order to effectively implement SSR programs into the community and to support healthy aging and “aging in place” post-hospitalization, evidence is needed to guide future SSR program model development and implementation. As well, evidence related to which older-adult profiles would most benefit from SSR programs is also required to guide the referral process. Researchers, health professionals, and government need to come together to develop a common understanding of – and language related to – SSR and expectations of SSR models of care.
Limitations
A common limitation of scoping reviews, including ours, is that although efforts were made to conduct a thorough scan of both empirical and grey literature, it is possible that not all relevant literature documents were identified in our search process. In order to define and assess SSR programs, we narrowed the search terms to literature documents that explicitly defined their rehabilitation as slow stream or long duration and low intensity. Older adult day programs, and day hospital programs that could have potentially been identified or classified as low intensity, long duration rehabilitation, but that did not define themselves as slow stream, were excluded. Thus, there is a possibility that this scoping review did not capture community-based programs or day hospitals that are using a similar model to programs but do not define themselves as SSR.
Furthermore, since we wanted to capture SSR programs in health care systems that were similar to those of Canada so that findings could be more readily integrated into our health care system, many countries with differing health care systems were excluded, such as the United States. Because the purpose of this scoping review was to obtain a broad understanding of the availability and research on SSR programs, we did not consider the quality of the literature and studies and did not assess it as part of the methodology.
Conclusion
Older adult patients, who are medically complex, cognitively impaired, and are considered to be of low rehabilitation potential, can make significant gains in both physical and ADL-related outcome measures through participating in a lower-intensity, longer-duration rehabilitation program. With further research, standardization of programs, standardization of referral processes, and integration of SSR programs into the community, SSR has the potential to be an integral part of Canada’s health care system. Although yet to be determined, community-based SSR may be economically beneficial and would provide opportunities to allow older adults with HAD and complex health and other needs to adjust to community living. Participating in lower intensity and longer duration rehabilitation (slow stream) upon returning to the community may also result in decreased hospital re-admission rates and decrease institutionalization.