No CrossRef data available.
Published online by Cambridge University Press: 09 July 2018
The chemiphoresis of montmorillonite saturated with Na, K, Li or Ca was studied in a system where corrosive dissolution of Al, Fe, Zn or Cu metal plates took place in the presence of HF and H2O2. The deposition of the clay as well as metal dissolution were found to be a function of 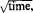 indicating that the electrochemical reaction is controlled principally by diffusion of the reacting species to the metal-liquid interface. The deposition increased with clay concentration and was lower for Ca-clay than for Na-, K- or Li-clay. It is shown that chemiphoresis alone does not provide sufficient data for the calculation of particle mobility and that other types of measurement are necessary. A relationship between particle deposition and electrical conductivity was developed, and examined experimentally with satisfactory results.
indicating that the electrochemical reaction is controlled principally by diffusion of the reacting species to the metal-liquid interface. The deposition increased with clay concentration and was lower for Ca-clay than for Na-, K- or Li-clay. It is shown that chemiphoresis alone does not provide sufficient data for the calculation of particle mobility and that other types of measurement are necessary. A relationship between particle deposition and electrical conductivity was developed, and examined experimentally with satisfactory results.