Article contents
Interactions of ammonium-smectite with volatile organic compounds from leachates
Published online by Cambridge University Press: 02 January 2018
Abstract
The percolation of water through waste landfills produces leachates with high concentrations of 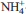 which can generate
which can generate 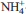 -exchanged clays within geochemical barriers. These leachates also contain several volatile organic compounds (VOCs) which can interact with the clay barrier. The aim of the present study was to characterize the sorption of eight short-chain VOCs (acetonitrile, methyl tert-butyl ether, dichloromethane, benzene, phenol, ethanol, acetone and aniline) on
-exchanged clays within geochemical barriers. These leachates also contain several volatile organic compounds (VOCs) which can interact with the clay barrier. The aim of the present study was to characterize the sorption of eight short-chain VOCs (acetonitrile, methyl tert-butyl ether, dichloromethane, benzene, phenol, ethanol, acetone and aniline) on 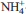 -smectite, and to identify their sorption mechanisms. The samples treated were characterized by carbon and nitrogen elemental analysis, infrared spectroscopy, powder X-ray diffraction, and thermogravimetric analysis. For acetonitrile, methyl tert-butyl ether, dichloromethane and benzene, no sorption was detected. Phenol, ethanol and acetone were sorbed very weakly, through Van der Waals interactions. Aniline molecules were sorbed strongly on
-smectite, and to identify their sorption mechanisms. The samples treated were characterized by carbon and nitrogen elemental analysis, infrared spectroscopy, powder X-ray diffraction, and thermogravimetric analysis. For acetonitrile, methyl tert-butyl ether, dichloromethane and benzene, no sorption was detected. Phenol, ethanol and acetone were sorbed very weakly, through Van der Waals interactions. Aniline molecules were sorbed strongly on 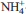 -smectite mainly with hydrogen bonds between aniline and interlayer water molecules. However, aniline sorption decreased the hydrophilic character of the
-smectite mainly with hydrogen bonds between aniline and interlayer water molecules. However, aniline sorption decreased the hydrophilic character of the 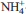 -smectite, which may increase the permeability of the clay barrier.
-smectite, which may increase the permeability of the clay barrier.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2017
References
- 4
- Cited by


