Introduction
Palygorskite is a fibrous clay mineral containing ribbons of 2:1 phyllosilicate structure, where the two continuous tetrahedral sheets show periodic inversion linked by one discontinuous octahedral sheet. The sandwiched octahedral sheets are interrupted periodically at the space where the two planes of tetrahedral sheets covered by basal oxygen are opposite, which endows palygorskite with plentiful nanopores and zeolite-like channels. The octahedral sheets of the perfect crystalline structure of palygorskite are occupied by Mg2+, while Al3+ and Fe3+ commonly occupy the octahedral sites of natural palygorskite as isomorphic substitutions. The terminal cations of discontinuous octahedral sheets are coordinated by water molecules (Bradley Reference Bradley1940; Chisholm Reference Chisholm1990, Reference Chisholm1992; Galán and Carretero Reference Galán and Carretero1999; Chryssikos et al. Reference Chryssikos, Gionis, Kacandes, Stathopoulou, Suárez and García-Romero2009). Palygorskite has many applications in paint, pharmacy, cosmetics, catalysis, nanocomposites, and environmental protection derived from its sorptive, rheological, and catalytic properties due to its special crystalline structure, chemical compositions, and fibrous morphology (Galán Reference Galán1996; Murray Reference Murray2000; Álvarez et al. Reference Álvarez, Santarén, Esteban-Cubillo, Aparicio, Galán and Singer2011; Ruiz-Hitzky et al. Reference Ruiz-Hitzky, Darder, Fernandes, Wicklein, Alcantara and Aranda2013; Wang and Wang Reference Wang and Wang2016).
Palygorskite clays of various geneses have different mineralogical and geochemical characteristics and physicochemical properties, which can influence their potential applications. The palygorskite clay deposited in Xuyi County, Jiangsu Province and Mingguang City, Anhui Province, China, for example, was transformed from the basaltic volcanic ash in a lacustrine environment; this palygorskite is very pure, has excellent dispersion viscosity, and can be used as both gelling grade and absorbent grade for various industrial applications in salt-formation drilling, paints, molecular sieves, decoloration of oils, etc. (Zhou and Murray Reference Zhou, Murray, Galán and Singer2011). Some palygorskite clays deposited in northwestern China were transformed from detrital clay minerals in a lacustrine environment and contain relatively large amounts of impurities, but are rich in trace elements such as Zn, Sr, B, and Se which are beneficial to livestock and crops. Feed and fertilizer incorporating this kind of palygorskite clay, for example, can increase the laying rate of laying hens and the production of wheat, potato, and oil-seed rape (Chen and Lin Reference Chen and Lin2019). Thus, they are suitable for applications in animal husbandry and plantation. Commercial deposits of palygorskite are rare compared to other laminar clay minerals such as montmorillonite and kaolinite, due to the particular environmental conditions of aridity and alkalinity required for the formation of palygorskite (Galán Reference Galán1996). Palygorskite is found mainly in soils and sediments of continental and marine environments in arid or semiarid climates (Callen Reference Callen, Singer and Galán1984; Galán and Pozo Reference Galán, Pozo, Galán and Singer2011; Hojati and Khademi Reference Hojati, Khademi, Gàlan and Singer2011), and it can also be the hydrothermal alteration product of igneous rocks (Stephen Reference Stephen1954; Nathan et al. Reference Nathan, Bentor and Wurtzburger1970; Furbish and Sando Reference Furbish and Sando1976; Suárez et al. Reference Suárez, García-Rivas, Sánchez-Migallón and García-Romero2018). The occurrence and genesis of palygorskite in calcretes and desert soils have been investigated by numerous researchers (Verrecchia and Le Coustumer Reference Verrecchia and Le Coustumer1996; Elidrissi et al. Reference Elidrissi, Daoudi and Fagel2018; Kadir et al. Reference Kadir, Eren, Külah, Erkoyun, Huggett and Önalgil2018) (and references therein). Many studies have shown that the palygorskite associated with pedogenic calcretes is of authigenic origin (Singer Reference Singer1979; Verrecchia and Le Coustumer Reference Verrecchia and Le Coustumer1996; Kadir et al. Reference Kadir, Eren, Kuelah, Oenalgil, Cesur and Guerel2014; Kaplan et al. Reference Kaplan, Muhsin, Kadir, Kapur and Huggett2014). In arid soils, besides the pedogenic authigenesis, palygorskite is also inherited from parent rocks during soil formation or brought from sources by sedimentary processes (alluvial or aeolian) (Lee et al. Reference Lee, Dixon and Aba-Husayn1983; Al-Bakri et al. Reference Al-Bakri, Khalaf and Al-Ghadban1984; Shadfan and Mashhady Reference Shadfan and Mashhady1985; Badraoui et al. Reference Badraoui, Bloom and Bouabid1992; Hameed et al. Reference Hameed, Raja, Ali, Upreti, Kumar and Tripathi2018). The palygorskite in the continental environment can be formed by direct precipitation from solution and through diagenetic reactions between solutions and preexisting minerals (Galán and Pozo Reference Galán, Pozo, Galán and Singer2011; Draidia et al. Reference Draidia, El Ouahabi, Daoudi, Havenith and Fagel2016; Kadir et al. Reference Kadir, Eren, Irkec, Erkoyun, Kulah and Onalgil2017). The release of Al, Mg, Si, and Fe from detrital clay minerals due to weathering and the subsequent recrystallization of these elements to form palygorskite have been reported widely (Couture Reference Couture1977; Tazaki et al. Reference Tazaki, Fyfe, Tsuji and Katayama1987; Chahi et al. Reference Chahi, Duplay and Lucas1993; Galán and Ferrero Reference Galán and Ferrero1982). Palygorskite formed by direct precipitation from solution was also proposed based on its morphology and the absence of other detrital clay minerals in the deep sea (Bowles et al. Reference Bowles, Angino, Hosterman and Galle1971; Thiry and Pletsch Reference Thiry, Pletsch, Gàlan and Singer2011). High Si, Mg, and low Al activities in alkaline media under arid and semiarid climates are prerequisites for precipitation of most authigenic palygorskites.
In previous studies, evidence for an authigenic origin of palygorskite transformed from detrital phyllosilicates was derived mainly from observation of the mineral texture. The observation of palygorskite fibers growing at the edge of smectite crystals is direct evidence of palygorskite transformed from pre-existing clay minerals (Rodas et al. Reference Rodas, Luque, Mas and Garzon1994; Xie et al. Reference Xie, Chen, Zhou, Xu, Xu and Ji2013; Kaplan et al. Reference Kaplan, Muhsin, Kadir, Kapur and Huggett2014). Some studies have used specific trace elements such as Rare-earth elements (REE) and transition elements as the discriminating parameter to trace the origin of palygorskite (Torres-Ruíz et al. Reference Torres-Ruíz, López-Galindo, González-López and Delgado1994; Lopez-Galindo et al. Reference Lopez-Galindo, Ben Aboud, Fenoll Hach-Ali and Casas Ruiz1996; Jamoussi et al. Reference Jamoussi, Ben Aboud and López-Galindo2003; Ye et al. Reference Ye, Yang, Fang, Hong, Zhang and Yang2018). Clay minerals formed by weathering or transformation tend to inherit some trace elements such as REE of their source rocks or minerals. The directly precipitated clay minerals in natural fresh water have very small REE contents compared to those transformed from other clay minerals (Torres-Ruíz et al. Reference Torres-Ruíz, López-Galindo, González-López and Delgado1994; Lopez-Galindo et al. Reference Lopez-Galindo, Ben Aboud, Fenoll Hach-Ali and Casas Ruiz1996; Jamoussi et al. Reference Jamoussi, Ben Aboud and López-Galindo2003). Separating palygorskite from other detrital clay minerals in one sample is impractical, as is measuring their trace elements separately. Thus, statistical analyses, including linear correlation analysis, cluster analysis, and factor analysis of principal components, were applied to reveal the occurrence of REE and enriched trace elements in palygorskite clays.
In the Yangtaiwatan basin, located in the middle of the Hexi Corridor, Gansu Province, China, palygorskite-rich mudstone interbedded with gypsum occurs in the Neogene Baiyanghe Formation. The mineralogical and geochemical characteristics of palygorskite-rich mudstone and the genesis of palygorskite have not been investigated systematically, however. The objective of the present study was, therefore, to determine whether or not palygorskite was of authigenic origin, transformed from a detrital laminar clay-mineral precursor, by investigating the mineralogical characteristics, in particular the morphology and texture of palygorskite and detrital laminar clay minerals, and the enrichment of REE and other trace elements Cs, U, B, Li, Sb, Bi, and As in the palygorskite. Such correlations may be helpful for evaluating the application of palygorskite clays containing large concentrations of trace elements in animal husbandry and plantation.
GEOLOGICAL SETTING, SAMPLING, AND METHODS
The study area is located in the southwest part of the Yangtaiwatan basin (Fig. 1), which is one of several continental downfaulted basins developed since the Cretaceous as a consequence of uplift of the Longshou mountain belt in the middle of the Hexi Corridor, Gansu Province, China. The basement rocks in the Yangtaiwatan basin are the lower part of the Cretaceous Miaogou Formation, which consists of sandy conglomerate, sandstone, mudstone, and muddy limestone (Fig. 2). The Neogene Baiyanghe Formation is widespread and overlies unconformably the basement rocks in the basin. The rocks of the Neogene Baiyanghe Formation are also clastic rocks including sandy conglomerate, sandstone, and mudstone of fluvial and lacustrine origin. The interbedded palygorskite-rich mudstone and gypsum occur in the middle–upper part of the Baiyanghe Formation. The middle Sinian unit and Lower Cretaceous Miaogou Formation are distributed sporadically around the basin (Fig. 1). The dominant rock types of the middle Sinian unit are slate, silicified limestone, marble, and dolomite. Biotite granite and monzogranite formed in the Hercynian period crop out at the north boundary of the basin. In the Late Pleistocene, the Gujiajing anticline and subsidiary Zhengbeishan syncline formed in the basin during regional compressional tectonic activity (Wang et al. Reference Wang, Cui and Zhang2005).
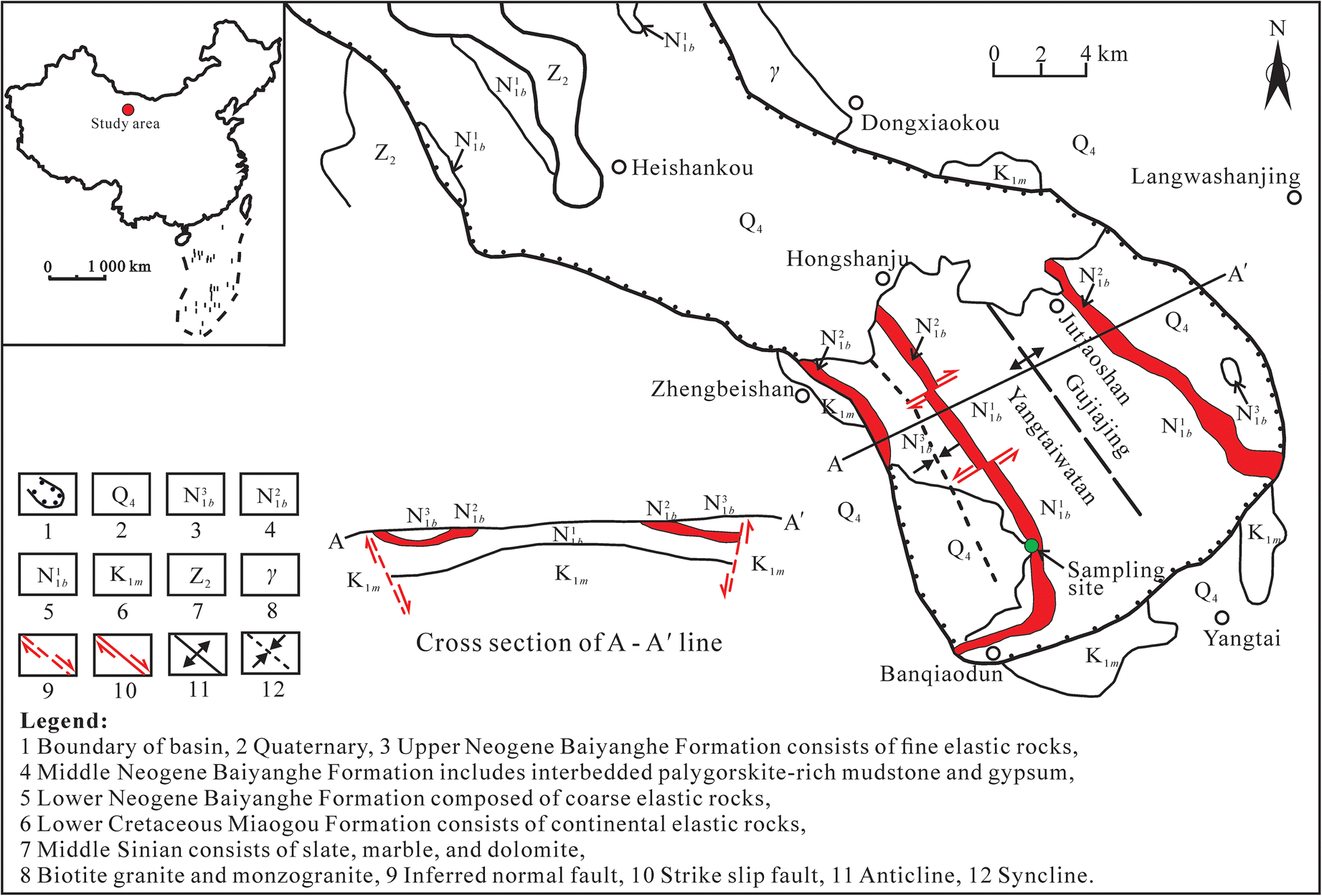
Fig. 1. Geological map of the Yangtaiwatan basin modified from Wang et al. (Reference Wang, Cui and Zhang2005)
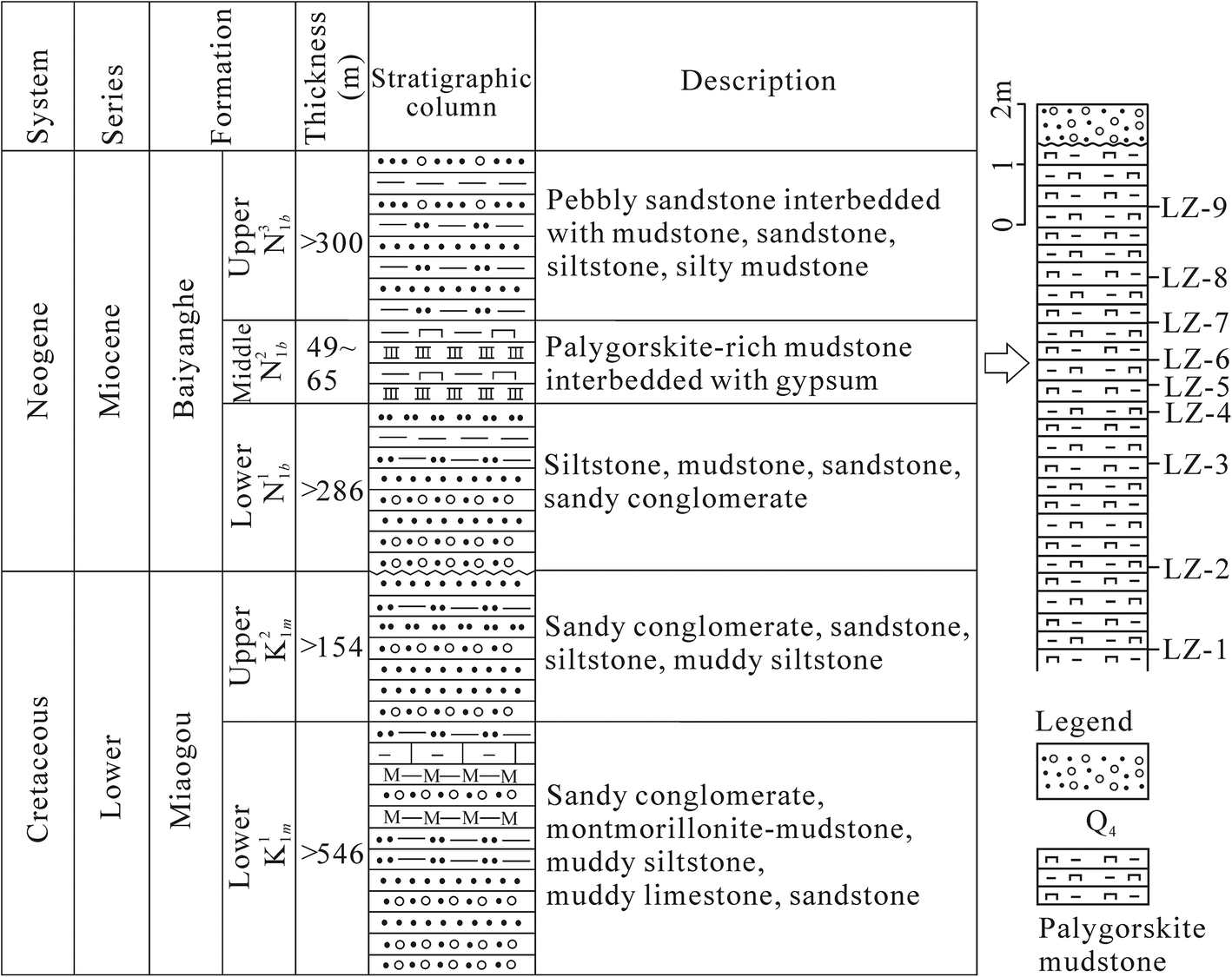
Fig. 2. Stratigraphic column of the Yangtaiwatan Basin modified from Wang et al. (Reference Wang, Cui and Zhang2005) and the sampling profile
Nine palygorskite-rich mudstone samples were collected from the bottom to the top of a field profile ~10 m thick, in the middle of the Baiyanghe Formation, located in the southwest part of the Yangtaiwatan basin (Figs 1 and 2). Several layers of gypsum <5 cm thick are interbedded in the palygorskite-rich mudstone exposed in the profile. The mineral composition of the bulk rock was determined using X-ray powder diffraction (XRD) on randomly oriented powders of samples scanned over the range of 2.5–70°2θ. For clay-mineral identification, the clay fractions of the bulk rocks were separated by sedimentation (Stokes’ law) and centrifugation of the suspension after 4 h of dispersion in distilled water. Oriented specimens of clay fractions were prepared by dripping the clay paste on the object slide and air drying. The oriented specimens of clay fractions were scanned firstly by XRD over the range 2.5–15°2θ. Then the oriented specimens were saturated with ethylene-glycol vapor for 8 h followed by scanning over the range 2.5–30°2θ. Lastly, the ethylene glycol-saturated oriented specimens were heated at 450°C for 2.5 h followed by scanning over the range 2.5–15°2θ. The XRD measurements were carried out using a Rigaku (Tokyo, Japan) D/max-2500PC diffractometer equipped with CuKα radiation operated at 40 kV and 100 mA at the State Key Laboratory of Coal Resources and Safe Mining, China University of Mining and Technology (Beijing). All XRD patterns were collected using the continuous sweep method at a scanning speed of 4°2θ/min. The sampling width was 0.02°2θ during the scanning. The quantitative analysis of mineral composition in the bulk rocks followed the method of Brindley (Reference Brindley, Brindley and Brown1980). The relative abundances of clay minerals were determined using their basal reflections and the mineral intensity factors of Moore and Reynolds (Reference Moore and Reynolds1989).
Based on the mineralogical characteristics of the bulk rock and clay fractions determined by XRD, scanning electron microscopy (SEM) observations were undertaken on freshly broken surfaces of representative bulk samples to investigate the morphology of minerals, especially the textural features of clay minerals and their relationship to other associated minerals. The freshly broken samples were stuck to aluminum sample holders by conductive colloidal silver, coated with a thin film of gold, and examined using an Hitachi (Tokyo, Japan) SU8020 field emission scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS) to determine the elemental contents for the areas selected during SEM observation. The SEM-EDS analysis was carried out at Beijing Zhongkebaice Technology Service Co., Ltd, China.
The chemical analyses were carried out at the Analytical Laboratory of Beijing Research Institute of Uranium Geology, China. The major elements were measured using a PANalytical (Almelo, Netherlands) Axios-mAX wavelength dispersive X-ray fluorescence spectrometer (XRF) according to the national standard method GB/T 14506.28-2010. The 44 trace elements (Li–Hf), including 15 rare earth elements (REE) and the trace element boron, were measured using an Element XR inductively coupled plasma source mass spectrometer (ICP-MS) (Thermo Fisher Scientific, Waltham, Massachusetts, USA) based on the standard methods GB/T 14506.30-2010 and HDB/T 3022-2018, respectively. The trace elements arsenic and selenium were determined using a LC-6500 atomic fluorescence spectrophotometer (AFS) (Beijing Haiguang Instrument Co., Ltd Beijing, China) following EJ/T 754-1993 and GB/T 22105.2-2008, respectively. (All of the standard methods are written in Chinese with English abstracts.)
Results
Mineralogical Characterization
The XRD patterns of bulk-rock samples (Fig. 3) revealed that they were composed mainly of quartz, feldspar (orthoclase and plagioclase), calcite, dolomite, gypsum, and clay minerals. The weight percentage of detrital quartz and feldspar varied from 10 to 25 wt.% and from 4 to 8 wt.%, respectively. Calcite and dolomite accounted for 2–20 wt.% and 3–15 wt.% of the bulk rocks, respectively. The neoformed gypsum made up 2–10 wt.%, and the clay minerals constituted >50 wt.% of the bulk rocks. The clay minerals separated from the bulk rocks contained mainly palygorskite and illite, the (110) and (001) reflections of which appeared in the range 8.6–8.8°2θ (Fig. 4). The accessory chlorite was identified by its (001) reflection with a d 001 value of 1.42 nm at 6.2°2θ. Kaolinite also occurred in the clay fraction, the (001) reflection of which coincided with the (002) reflection of chlorite at 12.4 2θ (Fig. 4). The separation of (002) reflection of kaolinite and (004) reflection of chlorite can be recognized at 25.0º2θ (Fig. 4). Palygorskite was the dominant clay mineral in all samples, accounting for 55–75 wt.% of the clay fractions in all samples. Illite constituted 20–30 wt.% of the clay fractions. The proportions of kaolinite and chlorite were similar (4–9 wt.%) in the clay fractions of all samples.
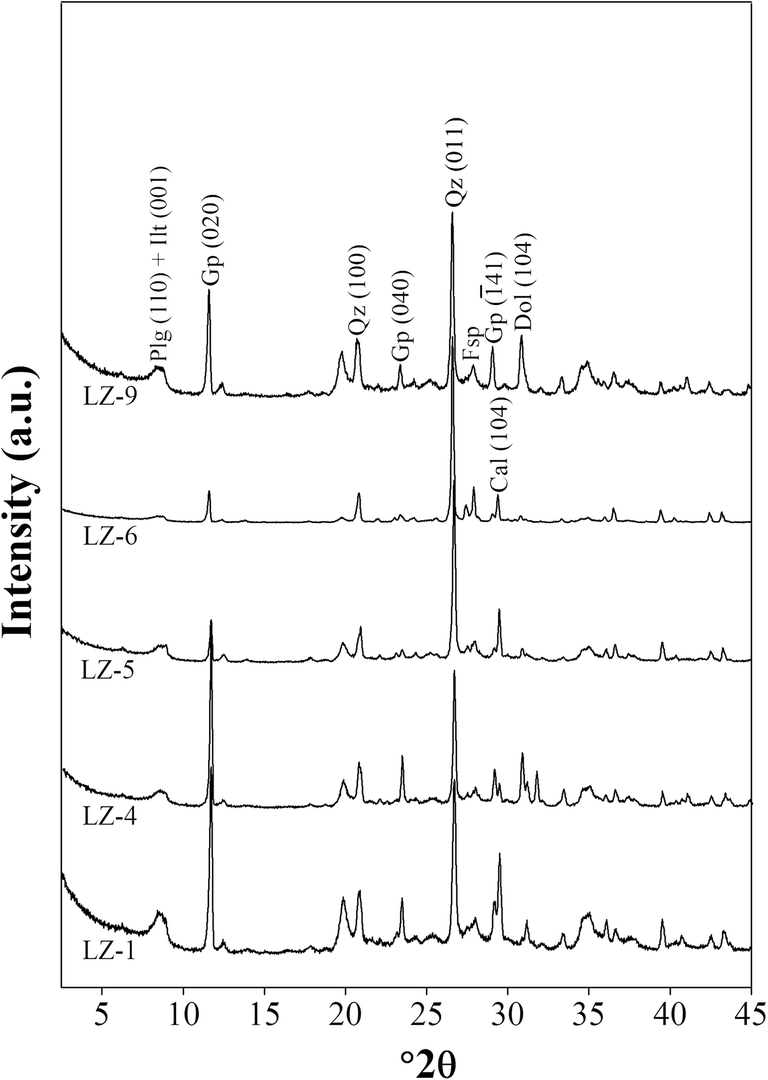
Fig. 3. XRD patterns of bulk-rock samples over the range 2.5–45°2θ. Plg: palygorskite, Ilt: illite, Gp: gypsum, Qz: quartz, Fsp: feldspar, Dol: dolomite, Cal: calcite
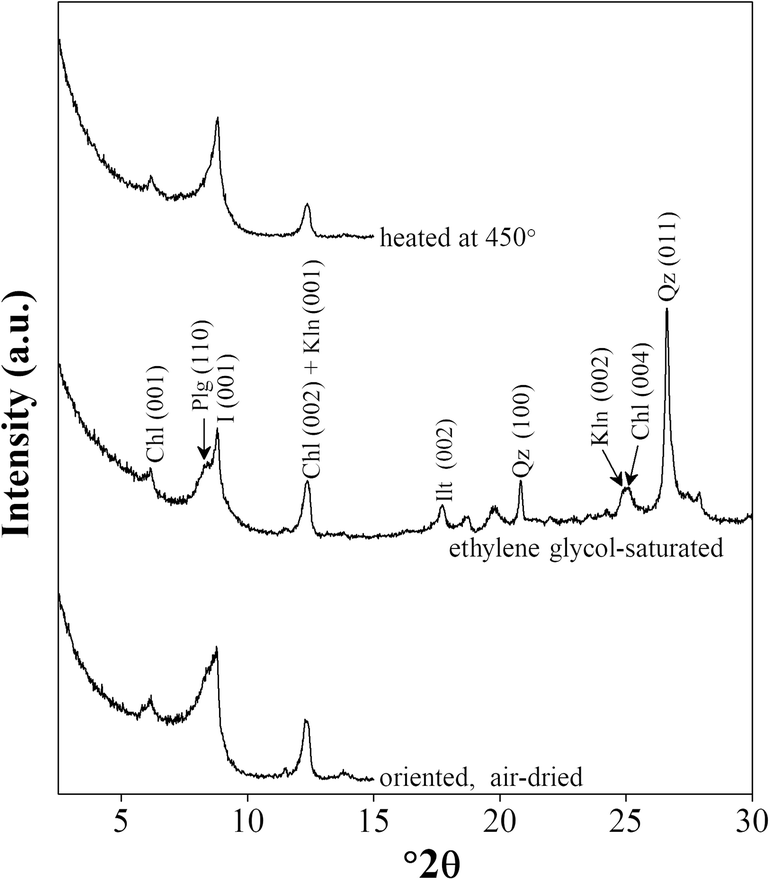
Fig. 4. XRD patterns of the clay fraction of sample LZ-9. Plg: palygorskite, Ilt: illite, Chl: chlorite, Kln: kaolinite
The SEM images of freshly broken surfaces of bulk-rock samples showed that palygorskite occurred as rod-like masses and interwoven between other minerals (Fig. 5a). Most of the palygorskite formed matted films and mixed with other laminar clay minerals as coatings on the detrital minerals (Fig. 5b, c, d, g, Fig. 6a), which made the detrital minerals difficult to recognize. The edges of laminar clay minerals were highly rounded. The EDS spectrum of a selected area (Fig. 6a) covering palygorksite and laminar clay minerals coating the detrital minerals with rounded edge exhibited a strong Si peak followed by Al, K, Mg, and Fe peaks, indicating the laminar clay minerals to be illite. The EDS spectrum of minerals in Fig. 6b showed a strong Ca peak followed by Si, Al, K, and Fe peaks, indicating that this mineral was calcite covered by illite. The outline of calcite was abraded, due mostly to the transportation process, indicating that the calcite was detrital in origin. Some palygorskite appeared to grow from the edge of laminar clay minerals (Fig. 5e, f), which suggested their close genetic relationship. The EDS analysis (Fig. 6c) showed the euhedral mineral with platy morphology had a chemical composition consisting mainly of Ca and S, corresponding to the chemical composition of gypsum. The accessory elements Si, Al, K, and Fe belonged to the illite coating on the surface of gypsum.

Fig. 5. SEM images of freshly broken surfaces of bulk-rock samples. a Rod-like palygorskite interwoven between other minerals; b, c, d palygorskite as matted films and mixed with other laminar clay minerals coating the detrital minerals; e, f palygorskite growing from the edge of laminar clay minerals; g palygorskite and other laminar clay minerals coating quartz; h magnified view of a corrosion hole in quartz in image g, showing that palygorskite grew in the corrosion hole. Legend as in Figs 3 and 4
Geochemical Characterization
The chemical analyses of bulk-rock samples including major elements, REE, and other trace elements are listed in Tables 1, 2, and 3. The major elements found were SiO2, Al2O3, CaO, and Fe2O3 + MgO + K2O corresponding to the presence of quartz, feldspar, calcite, gypsum, dolomite, palygorskite, and illite in the samples, which is consistent with results from XRD. The trace elements showed large variations compared to the average composition of the North American Shale Composite (NASC) (Gromet et al. Reference Gromet, Haskin, Korotev and Dymek1984) and the upper crust (UC) (Taylor and McLennan Reference Taylor and McLennan1985) as shown in Figs 7 and 8, respectively. Compared to trace elements of NASC, the samples were rich in Sr, Cs, and U. The mass ratios of these elements in samples compared to NASC were 2.1, 1.7, and 3.8, respectively. Compared to UC, the samples were rich in Li, Cr, Cd, Sb, Cs, Tl, Bi, U, As, and B. All the mass ratios were >2, especially the mass ratios of As and B, which reached 7.6 and 9.9, respectively. The samples were depleted in high field-strength elements Nb, Ta, Zr, and Hf that are associated with heavy minerals, such as zircon, which were in contrast to the enriched lithophile elements Li, Sr, Cs, and U.
Table 1. Major-element oxide compositions of bulk-rock samples (wt.%)
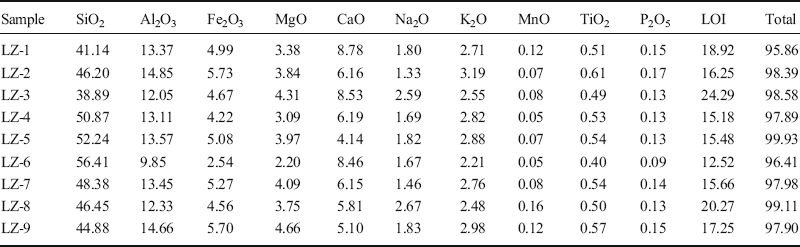
LOI: loss on ignition.
Table 2. REE of bulk-rock samples (ppm)
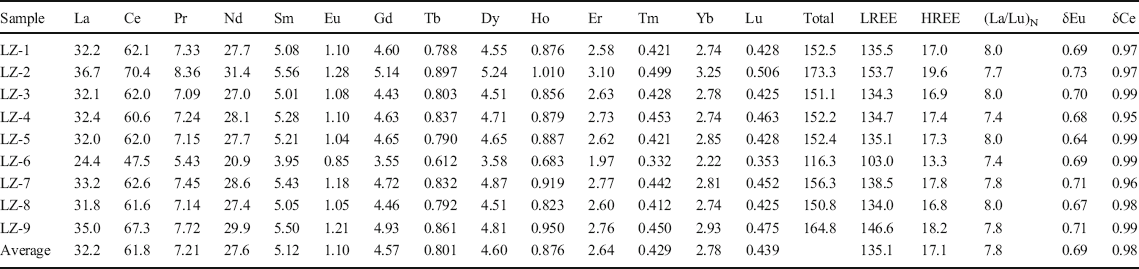
LREE is the sum of light REE, from La to Eu; HREE is the sum of heavy REE, from Gd to Lu. δEu = EuN/√(SmN × GdN), δCe = CeN/√(LaN × PrN), where the subscript N indicates normalization to chondrite.
Table 3. Trace-element composition of bulk-rock samples (ppm)
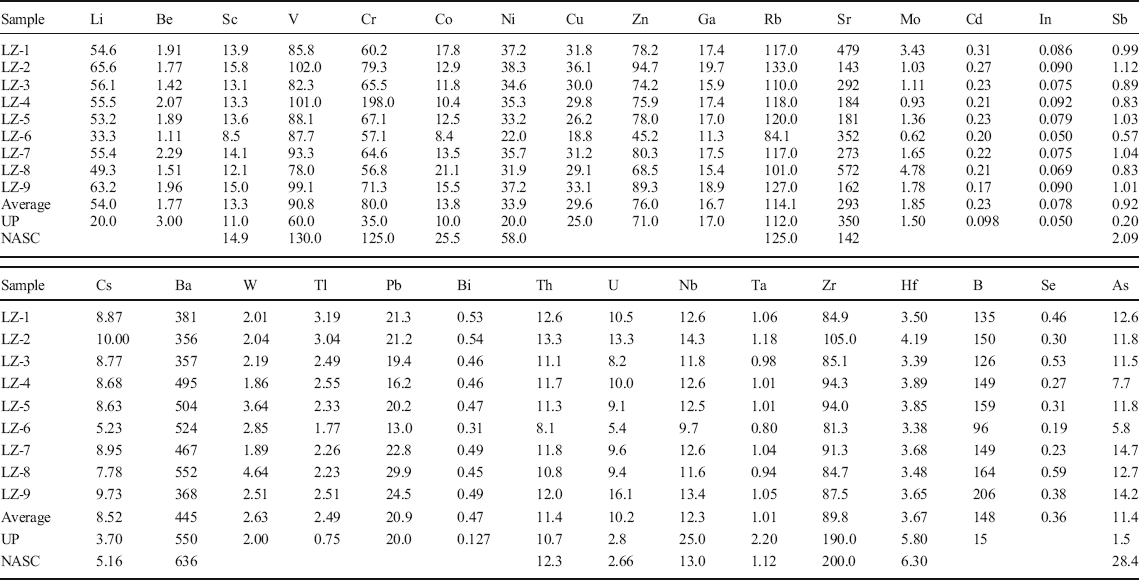
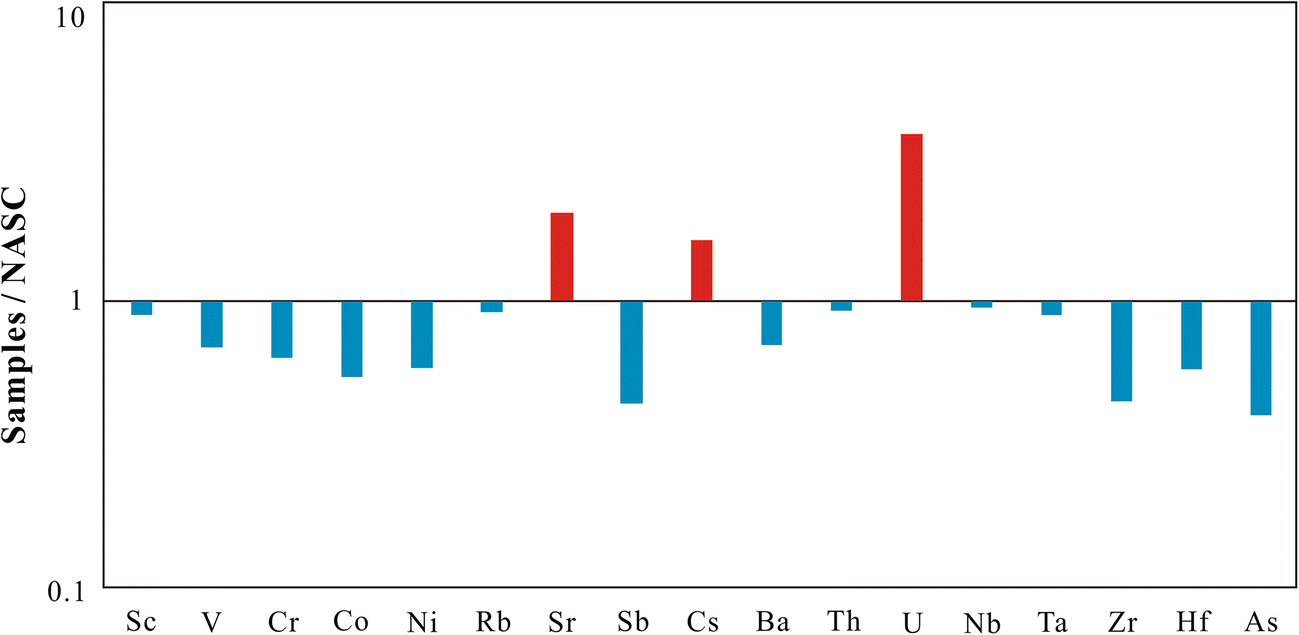
Fig. 7. Mass ratios of trace elements in samples compared with the North American Shale Composite (NASC)
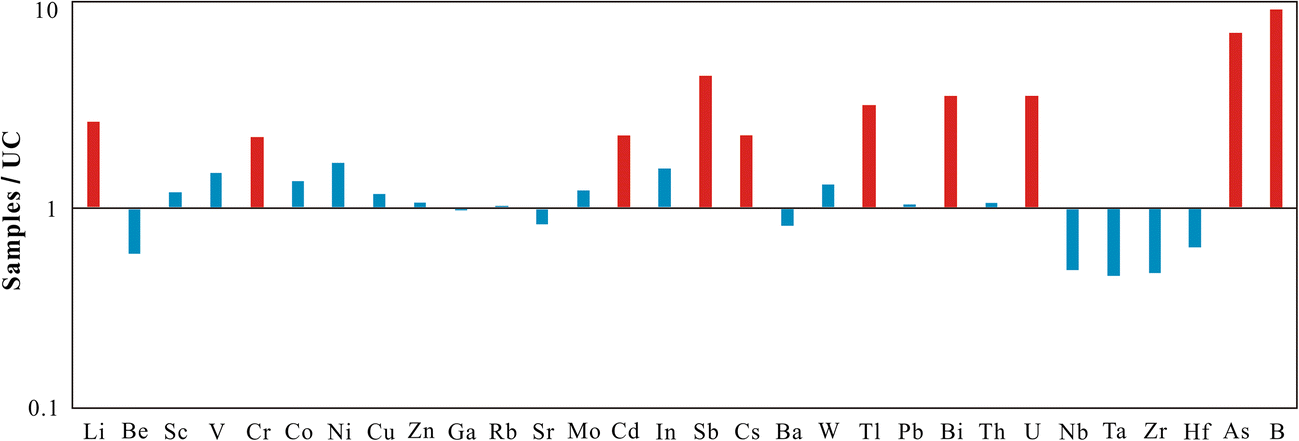
Fig. 8. Mass ratios of trace elements in samples compared to the upper crust (UC)
Analyses of the linear correlations between the major elements and the main enriched trace elements and REE (Table 4) revealed that the major and enriched trace elements made up clearly different groups according to their occurrence in minerals. High positive correlations were obtained among the major elements Al2O3, Fe2O3, MgO, K2O, and TiO2, which were the proxies for clay minerals as revealed by the EDS analyses of the minerals (Fig. 6a, b). High positive correlations were also found among the enriched trace elements Cs, U, B, Li, Sb, Bi, and As, and all of them correlated positively with Al2O3, Fe2O3, MgO, K2O, and TiO2 (Table 4). Strontium showed positive correlation with CaO and MnO only, with r values of 0.45 and 0.65, respectively. The major oxide SiO2 and enriched trace elements Cr and Cd did not show an obvious positive correlation with the other elements. The REE showed very positive correlations with Al2O3, Fe2O3, MgO, K2O, TiO2, Cs, U, B, Li, Sb, Bi, and As (Table 4). The cluster analysis of the major elements, REE, and main enriched trace elements based on their linear correlations (Fig. 9) showed the elements were divided into three groups. The Al2O3, K2O, TiO2, Cs, U, B, and Li were grouped together. The Fe2O3, MgO, Sb, Bi, and As were in another group. The third group included CaO, MnO, and As.
Table 4. Pearson correlation coefficients of the major-element oxides, REE, and main enriched trace elements
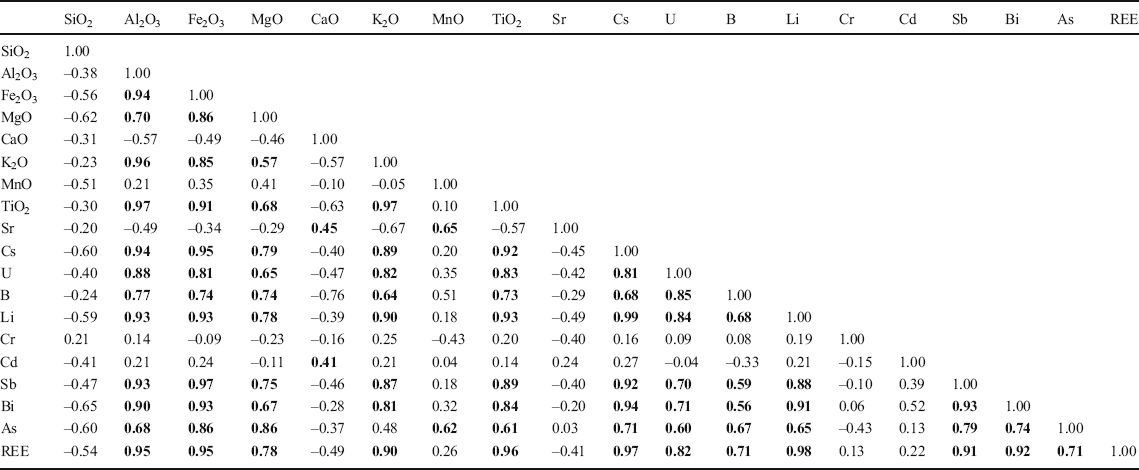
Values in bold are the significant correlation coefficients
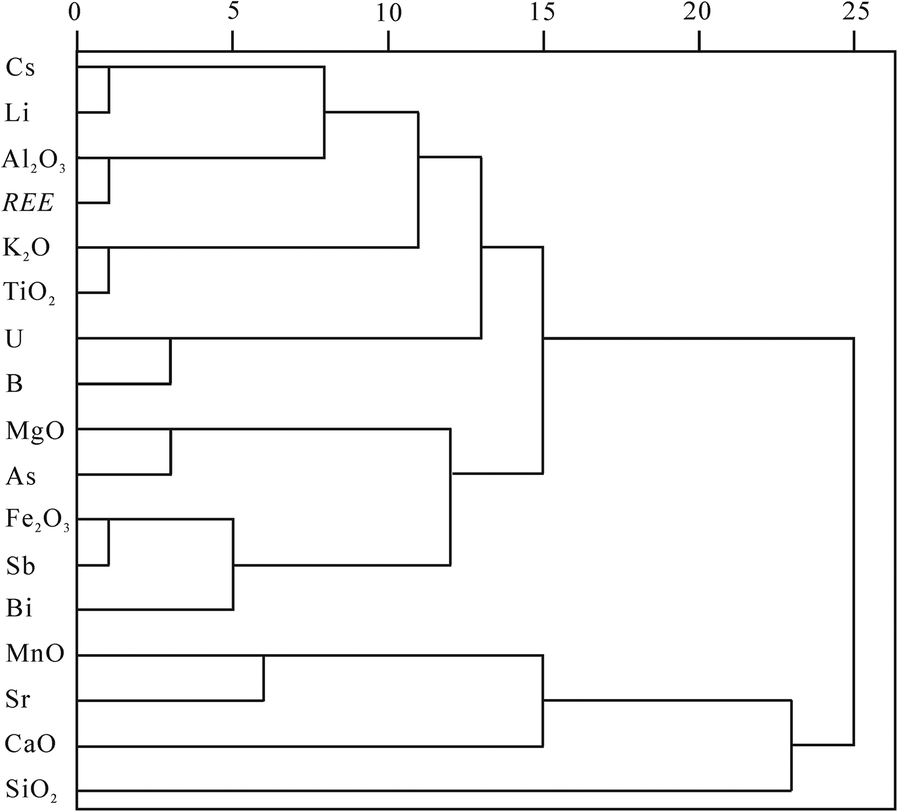
Fig. 9. Cluster analysis of the major-element oxides, REE, and main enriched trace elements of the samples
Factor analysis of principal components of the main minerals, major elements, REE, and enriched trace elements, found that three factors together explained 88.6% of the total variance within the data (Fig. 10). Factor 1 (60.5%) separated clearly the clay minerals and the associated elements from the detrital minerals quartz and feldspar and their associated elements. Factor 2 (18.4%) distinguished mainly the dolomite with associated elements and calcite with associated elements. Factor 3 (9.7%) separated gypsum and palygorskite from the detrital minerals such as quartz, feldspar, calcite, illite, kaolinite, and chlorite.
The concentrations of REE (Fig. 11) indicated that all samples showed similar chondrite-normalized (Boynton Reference Boynton1984) REE patterns. The light REE (LREE) were enriched relative to the heavy REE (HREE), which was similar to the chondrite-normalized patterns for shale in general (Nyakairu and Koeberl Reference Nyakairu and Koeberl2001). The chondrite-normalized La/Lu ratios were in the range 7.36–8.04 (Table 2). The relative depletion in the HREE compared to LREE may be due to the trace content of heavy minerals in the samples, which was also reflected by the depletion of high field-strength elements Nb, Ta, Zr, and Hf (Figs 7 and 8). All samples had negative Eu anomalies ranging from 0.64 to 0.73 (Table 2).
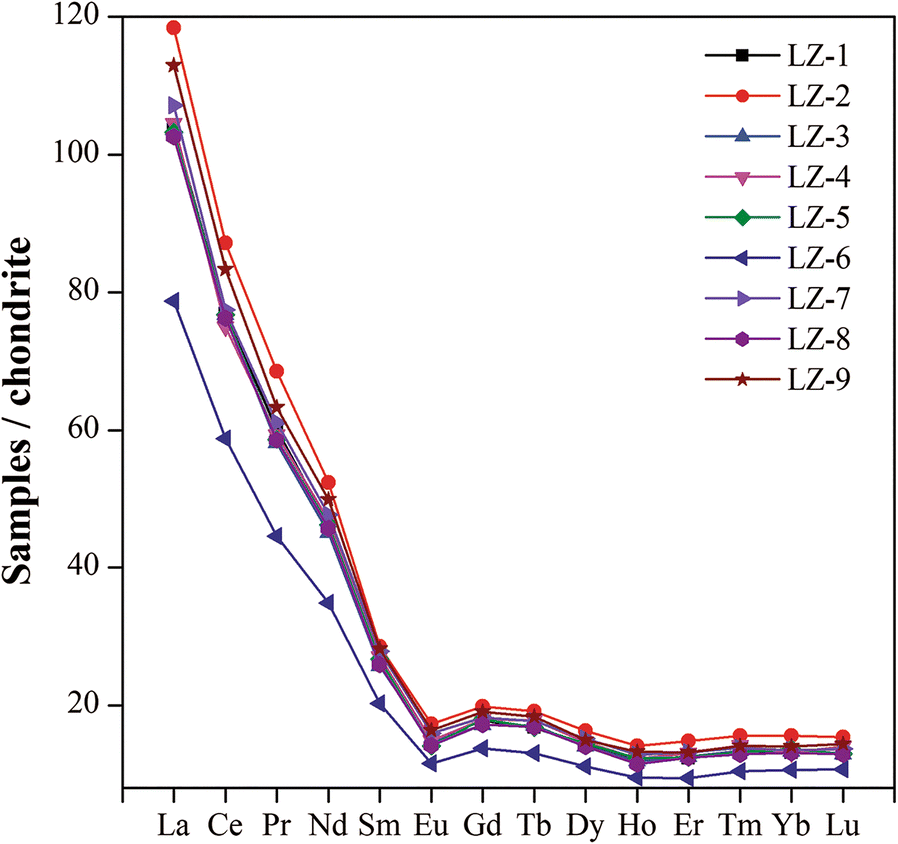
Fig. 11. Chondrite-normalized REE patterns of the samples
Discussion
The palygorskite was present in red- and green-colored lacustrine mudstones interbedded with gypsum in the Neogene Baiyanghe Formation. Gypsum crystallization and enrichment in Sr were also found in the palygorskite-rich mudstone (Figs 6 and 7). This suggested that the sediments were subjected to an arid climate (Inoue et al. Reference Inoue, Saito and Naruse1998). Such a climate led to the evaporative concentration of solutions and consequently an increase in pH and salinity. The enrichment of B in the samples (Fig. 8) supports the theory that the lacustrine sediments were deposited in a salty medium (Wang et al. Reference Wang, Lin, Wei, Zhou, Chang and Liu2017). These conditions were favorable to palygorskite formation.
In the SEM images, the palygorskite occurred as rod-like masses and interwoven between other minerals (Fig. 5a). The interwoven rod-like palygorskite aggregates and delicate palygorskite particles were unlikely to survive transportation and redeposition and, therefore, an authigenic origin is indicated. In addition, the palygorskite also grew on the edge of laminar clay minerals (Fig. 5e, f), suggesting a dissolution and precipitation mechanism as supersaturated conditions developed, similar to previous studies (Chahi et al. Reference Chahi, Duplay and Lucas1993; Kadir and Eren Reference Kadir and Eren2008; Tlili et al. Reference Tlili, Felhi and Montacer2010; Verrecchia and Le Coustumer Reference Verrecchia and Le Coustumer1996; Lopez-Galindo et al. Reference Lopez-Galindo, Ben Aboud, Fenoll Hach-Ali and Casas Ruiz1996). The weathering of clay minerals such as smectites and mixtures of illite, kaolinite, and chlorite, and recrystallization of palygorskite have been reported widely in calcretes, desert soils, and lacustrine deposits (Yaalon and Wieder Reference Yaalon and Wieder1976; Singer Reference Singer1979; Paquet Reference Paquet1983; Ingles and Anadon Reference Ingles and Anadon1991; Chahi et al. Reference Chahi, Duplay and Lucas1993; Verrecchia and Le Coustumer Reference Verrecchia and Le Coustumer1996; Kadir et al. Reference Kadir, Eren, Kuelah, Oenalgil, Cesur and Guerel2014) (and references therein). Arid or semi-arid climate, an alkaline medium, high Si and Mg activity, and low Al activity must, therefore, be the prerequisites for palygorskite formation.
The highly rounded edges of detrital laminar clay minerals (Fig. 5b, c, d, e, f) suggested that they were probably subjected to chemical weathering. According to the study by Paquet (Reference Paquet1983), phyllosilicates, are unstable under hyperalkaline conditions and tend to dissolve and release Al, Fe, Si, and Mg. Thus, the Al required for palygorskite precipitation in the lake was supplied mainly by the dissolution of these detrital clay minerals. Besides the Si and Mg input from the dissolution of detrital clay minerals, a certain amount of Si was provided by the weathering of quartz as revealed by the corrosion holes in quartz, where the palygorskite was precipitated (Fig. 5g, h). The Mg was probably provided by the weathering of detrital dolomite from the Middle Sinian unit at the northern boundary of the basin. The weathered dolomite was not recognized, however, in an examination using SEM, due to the coating of neoformed palygorskite and detrital laminar clay minerals. In a recent study (Ryan et al. Reference Ryan, Kaczmarek and Rivers2019), the dissolution of metastable dolomite was found to play an import role in the precipitation of palygorskite by buffering the pH of solution and releasing Mg into the solution. Weathered detrital calcite was observed under the SEM (Fig. 6b) and this was considered to be the main source of Ca in the lake.
Other important evidence supporting the idea that the elements of palygorskite were provided by the dissolution of detrital clay minerals was the occurrence of REE and enriched trace elements in the samples. As stated above, separating the palygorskite from other detrital clay minerals in any one sample and measuring their trace elements separately is impractical, so statistical analyses were undertaken. This included linear correlation analysis, cluster analysis, and factor analysis of principal components of the main minerals, major elements, REE, and enriched trace elements (Table 4, Figs 9 and 10), all of which helped to explain the occurrence of REE and enriched trace elements in the samples. The quartz accumulated practically no REE nor became enriched in trace elements, as revealed by the negative correlation of REE and enriched trace elements with SiO2 that was the proxy of quartz (Table 4). The REE and enriched trace elements, other than Sr and Cd, also showed negative correlation with CaO which was the proxy for calcite and gypsum (Table 4), indicating that the detrital calcite and neoformed gypsum also contained hardly any REE. The Sr was associated to a small extent with gypsum as revealed by the factor analysis of principal components (Factor 3) (Fig. 10), indicating that Sr was incorporated in gypsum during its precipitation with water evaporation in the arid climate. MgO as a proxy for palygorskite and dolomite showed good linear correlation with Al2O3, Fe2O3, K2O, TiO2, REE, and enriched trace elements Cs, U, B, Li, Sb, Bi, and As. According to the factor analysis of principal components (Factor 1) (Fig. 10), these elements were all associated with palygorskite rather than dolomite. Previous studies also showed that the calcite and dolomite contained few REE (Ronov et al. Reference Ronov, Balashov, Girin, Bratishko and Kazakov1974). The Al2O3 also showed very positive correlation with major elements Fe2O3, MgO, K2O, TiO2, REE, and the enriched trace elements listed above. Generally, the Al2O3 and K belonged to the basic elements of illite. The Al2O3 and MgO were the basic components of palygorskite. The SEM-EDS analyses (Fig. 6) showed that Fe and Ti as isomorphic substitutions were hosted in the lattice of illite and palygorskite. Thus, one concludes that the Cs, U, B, Li, Sb, Bi, and As were hosted in the detrital laminar clay minerals and neoformed palygorskite. Trace amounts of B have been reported to have been adsorbed mainly by illite, kaolinite, and smectite in a salty medium, which was commonly used for construction of paleosalinity (Adams et al. Reference Adams, Haynes and Walker1965; Couch Reference Couch1971). A previous study (Nyakairu and Koeberl Reference Nyakairu and Koeberl2001) also showed that the As and Sb were concentrated in samples that were rich in Fe2O3 due to weathering under arid oxidizing conditions. Linear correlation analysis and cluster analysis (Table 4 and Fig. 9) showed that the REE were highly correlated with Al2O3, Fe2O3, MgO, K2O, TiO2, and with the enriched trace elements above, suggesting that the clay minerals were also the carrier of REE. The factor analysis of principal components (Factor 1) (Fig. 10) showed that the REE and enriched trace elements listed above had strong affinity with palygorskite compared to illite, kaolinite, and chlorite due to the large proportion of neoformed palygorskite (55–75%) in the clay fractions. This indicated that the palygorskite was the main carrier of REE and the enriched trace elements. Another previous study (Torres-Ruíz et al. Reference Torres-Ruíz, López-Galindo, González-López and Delgado1994) showed that the directly precipitated clay minerals in natural fresh water had very small REE contents compared to that transformed from other clay minerals. Thus, the palygorskite apparently was formed by weathering or dissolution of detrital clay minerals as the original host of REE and enriched trace elements and recrystallization in an alkaline medium with an arid climate. The REE and enriched trace elements were inherited from the dissolved detrital clay minerals. Some REE and trace elements were also found occurring in the neoformed palygorskite formed from weathering of other clay minerals (Torres-Ruíz et al. Reference Torres-Ruíz, López-Galindo, González-López and Delgado1994; Lopez-Galindo et al. Reference Lopez-Galindo, Ben Aboud, Fenoll Hach-Ali and Casas Ruiz1996). The chondrite-normalized REE of all samples showed essentially the same pattern (Fig. 11) irrespective of the proportions of detrital illite, kaolinite, chlorite, and neoformed palygorskite that were present in the samples. This also meant that the generation of palygorskite originated from the dissolution of detrital clay minerals without loss of REE. The REE pattern of sample LZ-6 is low compared to other samples due to the smallest REE contents (Table 2) and clay minerals contents. The REE are hosted mainly in the clay minerals, thus the small amount of clay minerals results in the small REE contents.
Conclusions
The palygorskite occurring in the lacustrine mudstones of the Neogene Baiyanghe Formation in the Yangtaiwatan basin were of authigenic origin and were transformed from the weathering of detrital laminar clay minerals as indicated by their morphology and the state of occurrence of REE and enriched trace elements in the samples. The original detrital clay minerals underwent post-depositional weathering processes in an alkaline medium and arid climates, releasing the Al, Si, Mg, and Fe required for palygorskite formation, as shown by the enriched REE and other trace elements in neoformed palygorskite. The Mg and Si were also provided by the erosion of dolomite and quartz from the Middle Sinian and the Lower Cretaceous Miaogou Formation surrounding the basin. The Ca in the medium was input mainly by the weathering of calcite. The arid climate led to the concentration of solutions and consequently an increase in pH and salinity of the medium in the basin. The gypsum occurring as seams interbedded with mudstones and mineral composition in the bulk-rock of mudstones was precipitated by concentration of solution, resulting in an increase in the Mg/Ca ratio. The palygorskite was then precipitated, resulting in the crystallization of Si, Al, Mg, and Fe, and by incorporation or adsorption of REE and other trace elements released by dissolution of detrital laminar clay minerals.
ACKNOWLEDGMENTS
This work was supported financially by the National Natural Science Foundation of China (41802189), the Open Project on Development of Palygorskite Industry in Linze County, Gansu Province, China (LZKFKT-1801), and by the Fundamental Research Funds for the Central Universities (2020XJDC04).
Funding
Funding sources are as stated in the Acknowledgments.
Compliance with Ethical Statements
Conflict of Interest
The authors declare that they have no conflict of interest.



















