Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kuang, Wenxing
Facey, Glenn A.
and
Detellier, Christian
2004.
Dehydration and rehydration of palygorskite and the influence of water on the nanopores.
Clays and Clay Minerals,
Vol. 52,
Issue. 5,
p.
635.
García-Romero, E.
Suárez Barrios, M.
and
Bustillo Revuelta, M. A.
2004.
Characteristics of a Mg-palygorskite in Miocene rocks, Madrid Basin (Spain).
Clays and Clay Minerals,
Vol. 52,
Issue. 4,
p.
484.
Suárez, M.
and
García-Romero, E.
2006.
FTIR spectroscopic study of palygorskite: Influence of the composition of the octahedral sheet.
Applied Clay Science,
Vol. 31,
Issue. 1-2,
p.
154.
Bayram, H.
Önal, M.
Üstünışık, G.
and
Sarıkaya, Y.
2007.
Some thermal characteristics of a mineral mixture of palygorskite, metahalloysite, magnesite and dolomite.
Journal of Thermal Analysis and Calorimetry,
Vol. 89,
Issue. 1,
p.
169.
Post, James L.
and
Crawford, Susan
2007.
Varied forms of palygorskite and sepiolite from different geologic systems.
Applied Clay Science,
Vol. 36,
Issue. 4,
p.
232.
García-Romero, E.
Suárez, M.
Santarén, J.
and
Alvarez, A.
2007.
Crystallochemical characterization of the palygorskite and sepiolite from the Allou Kagne deposit, Senegal.
Clays and Clay Minerals,
Vol. 55,
Issue. 6,
p.
606.
Cai, Yuanfeng
Xue, Jiyue
and
Polya, D.A.
2007.
A Fourier transform infrared spectroscopic study of Mg-rich, Mg-poor and acid leached palygorskites.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 66,
Issue. 2,
p.
282.
Gionis, Vassilis
Kacandes, George H.
Kastritis, Ioannis D.
and
Chryssikos, Georgios D.
2007.
Combined near-infrared and X-ray diffraction investigation of the octahedral sheet composition of palygorskite.
Clays and Clay Minerals,
Vol. 55,
Issue. 6,
p.
543.
Huang, Yan-Jun
Li, Zhen
Li, Shu-Zhen
Shi, Zi-Liang
Yin, Lin
and
Hsia, Yuan-Fu
2007.
Mössbauer investigations of palygorskite from Xuyi, China.
Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms,
Vol. 260,
Issue. 2,
p.
657.
Li, Z.
He, K.
Yin, L.
Xiong, F.
and
Zheng, Y. C.
2007.
Crystallochemistry of Fe-rich palygorskite from eastern China.
Clay Minerals,
Vol. 42,
Issue. 4,
p.
453.
Tao, CHEN
Hejing, WANG
Xiaoping, ZHANG
and
Nan, ZHENG
2008.
SAED and HRTEM Investigation of Palygorskite.
Acta Geologica Sinica - English Edition,
Vol. 82,
Issue. 2,
p.
385.
Burzo, E.
2009.
Phyllosilicates.
Vol. 27I5b,
Issue. ,
p.
444.
Boudriche, L.
Hamdi, B.
Kessaïssia, Z.
Calvet, R.
Chamayou, A.
Dodds, J. A.
and
Balard, H.
2010.
An Assessment of the Surface Properties of Milled Attapulgite Using Inverse Gas Chromatography.
Clays and Clay Minerals,
Vol. 58,
Issue. 2,
p.
143.
Garciá-Romero, E.
and
Suárez, M.
2010.
On the Chemical Composition of Sepiolite and Palygorskite.
Clays and Clay Minerals,
Vol. 58,
Issue. 1,
p.
1.
Frost, Ray L.
Xi, Yunfei
and
He, Hongping
2010.
Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption.
Journal of Colloid and Interface Science,
Vol. 341,
Issue. 1,
p.
153.
Madejová, Jana
Balan, Etienne
and
Petit, Sabine
2010.
Advances in the characterization of industrial minerals.
p.
171.
Frankovská, J.
Andrejkovičová, S.
and
Janotka, I.
2010.
Effect of NaCl on hydraulic properties of bentonite and bentonite–palygorskite mixture.
Geosynthetics International,
Vol. 17,
Issue. 4,
p.
250.
Cheng, Hongfei
Yang, Jing
Frost, Ray L.
and
Wu, Zeguang
2011.
Infrared transmission and emission spectroscopic study of selected Chinese palygorskites.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 83,
Issue. 1,
p.
518.
Bouna, L.
Rhouta, B.
Amjoud, M.
Maury, F.
Lafont, M.-C.
Jada, A.
Senocq, F.
and
Daoudi, L.
2011.
Synthesis, characterization and photocatalytic activity of TiO2 supported natural palygorskite microfibers.
Applied Clay Science,
Vol. 52,
Issue. 3,
p.
301.
Yin, Hui
Liu, Fan
Feng, Xionghan
Liu, Mingming
Tan, Wenfeng
and
Qiu, Guohong
2011.
Co2+-exchange mechanism of birnessite and its application for the removal of Pb2+ and As(III).
Journal of Hazardous Materials,
Vol. 196,
Issue. ,
p.
318.
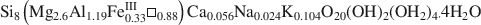 , was studied by FTIR spectroscopy. In both OH-stretching and OH-bending regions, there is evidence of dioctahedral Al2□OH, AlFe□OH and trioctahedral Mg3OH features, leading to a di-trioctahedral crystallochemical model of octahedral site occupancies in ribbons of Ganntour palygorskite.
, was studied by FTIR spectroscopy. In both OH-stretching and OH-bending regions, there is evidence of dioctahedral Al2□OH, AlFe□OH and trioctahedral Mg3OH features, leading to a di-trioctahedral crystallochemical model of octahedral site occupancies in ribbons of Ganntour palygorskite.