Published online by Cambridge University Press: 01 July 2024
The formation of an interstratified structure in dioctahedral smectite was assumed to be influenced by (1) the overall layer charge density and its distribution in the structure, (2) the solvation energy of the cation, and (3) the nature of the solvation agent. By holding factors (2) and (3) constant it was possible to calculate the average local charge densities $\overline {{\rm{QA}}}$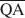 , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $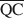 , and $\overline {{\rm{QE}}} $
, and $\overline {{\rm{QE}}} $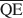 which are necessary for formation of 10-, 14-, and 16.8-Å mixed-layer phases in potassium-treated and ethylene glycol (EG) saturated smectites. The values of $\overline {{\rm{QA}}} $
which are necessary for formation of 10-, 14-, and 16.8-Å mixed-layer phases in potassium-treated and ethylene glycol (EG) saturated smectites. The values of $\overline {{\rm{QA}}} $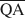 , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $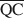 , and $\overline {{\rm{QE}}} $
, and $\overline {{\rm{QE}}} $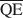 were 1.99, 1.2, and 0.56 esu/unit cell, respectively. Ammonium-treated smectites saturated with EG gave corresponding mean local charge densities of 2.7, 1.6, and 0.72 esu/unit cell. Calculations were made under the limiting condition QA > QC > QE > 0.
were 1.99, 1.2, and 0.56 esu/unit cell, respectively. Ammonium-treated smectites saturated with EG gave corresponding mean local charge densities of 2.7, 1.6, and 0.72 esu/unit cell. Calculations were made under the limiting condition QA > QC > QE > 0.
For K-smectites saturated with EG, Qtot = 1.99pA + 1.2pC + 0.56pE, where Qtot is the total charge (esu/unit cell), and pA, pC, and pE are probability coefficients for 10-, 14-, and 16.8-Å phases in the interstratified structure. The above equation calculated with the aid of least squares and without the limiting condition yields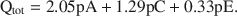
Предполагается, что на образование внутринапластованной структуры в диоктаедрическом смектите влияют: (1) полная плотность заряда слоя и её распределение в структуре, (2) энергия сольватации катиона, и (3) природа агента сольватации. Поддерживая факторы (2) и (3) постоянными, можно рассчитать средние местные плотности заряда $\overline {{\rm{QA}}}$ , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $ , и $\overline {{\rm{QE}}} $
, и $\overline {{\rm{QE}}} $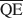 , необходимые для образования 10-, 14-, и 16,8-А фаз со смешанными слоями в смектите, обработанном потасом и насыщенном этиленовым гликолем (ЭГ). Величины $\overline {{\rm{QA}}} $
, необходимые для образования 10-, 14-, и 16,8-А фаз со смешанными слоями в смектите, обработанном потасом и насыщенном этиленовым гликолем (ЭГ). Величины $\overline {{\rm{QA}}} $ , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $ , и $\overline {{\rm{QE}}} $
, и $\overline {{\rm{QE}}} $ , были 1,99, 1,2 и 0,56 эе/эя (электростатическая единица/элементарная ячейка), соответственно. Для смектитов, обработанных аммонием, насыщенных ЭГ, соответствующие средние местные плотности заряда были 2,7, 1,6, и 0,72 эе/эя. Были проведены расчеты при условии: QА > QС > QЕ > 0.
, были 1,99, 1,2 и 0,56 эе/эя (электростатическая единица/элементарная ячейка), соответственно. Для смектитов, обработанных аммонием, насыщенных ЭГ, соответствующие средние местные плотности заряда были 2,7, 1,6, и 0,72 эе/эя. Были проведены расчеты при условии: QА > QС > QЕ > 0.
Для К-смектитов, насыщенных ЭГ, Qпол = 1,99рА + 1,2рС + 0,56рЕ, где: Qпол = полный заряд; рА, рС, и рЕ = коеффициенты вероятности для 10-, 14-, и 16,8-Å фаз во внутринапластованной структуре. Вышеупомянутое уравнение, решенное при помощи метода наиментших квадратов и без ограничивающего условия, имеет вид: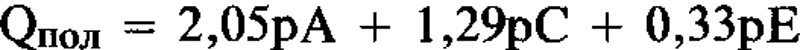 Наблюдается хорошее соответствие между величинами слойпого заряда для К-смектита и величинами для слюды, вермикулита и монтмориллонита, для которых вышеупомянутые расстояния элементарной структуры явлются типичными. [Е.С.]
Наблюдается хорошее соответствие между величинами слойпого заряда для К-смектита и величинами для слюды, вермикулита и монтмориллонита, для которых вышеупомянутые расстояния элементарной структуры явлются типичными. [Е.С.]
Es wurde angenommen, daß die Bildung einer Wechsellagerungsstruktur in dioktaedrischem Smektit beeinflußt wird durch (1) die gesamte Schichtladungsdichte und ihre Verteilung in der Struktur, (2) die Solvatationsenergie des Kations, und (3) die Art des Lösungsmittels. Indem die Faktoren (2) und (3) konstant gehalten wurden, war es möglich die lokalen durchschnittlichen Ladungsdichten$\overline {{\rm{QA}}} $ , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $ , und $\overline {{\rm{QE}}} $
, und $\overline {{\rm{QE}}} $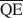 zu berechnen, die für die Bildung von 10-, 14- und 16.8-Å Wechsellagerungsphasen in Kalium-behan-delten und Ethylenglycol (EG)-gesätttigten Smektiten notwendig sind. Die werte von $\overline {{\rm{QA}}} $
zu berechnen, die für die Bildung von 10-, 14- und 16.8-Å Wechsellagerungsphasen in Kalium-behan-delten und Ethylenglycol (EG)-gesätttigten Smektiten notwendig sind. Die werte von $\overline {{\rm{QA}}} $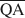 , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $ , und $\overline {{\rm{QE}}} $
, und $\overline {{\rm{QE}}} $ betrugen 1,99, 1,2 und 0,56 esu/uc, bzw. Ammonium-behandelte, EG-gesättigte Smektite gaben entsprechende mittlere lokale Ladungsdichten von 2,7, 1,6, und 0,72 esu/uc. Es wurden Berechnungen durchgeführt mit der Einschränkung QA > QC > QE > 0.
betrugen 1,99, 1,2 und 0,56 esu/uc, bzw. Ammonium-behandelte, EG-gesättigte Smektite gaben entsprechende mittlere lokale Ladungsdichten von 2,7, 1,6, und 0,72 esu/uc. Es wurden Berechnungen durchgeführt mit der Einschränkung QA > QC > QE > 0.
Bei EG-gesättigtem K-Smektit ergab sich Qtot = 1,99pA + 1,2pC + 0,56pE, wobei Qtot die Gesamtladung (esu/uc) ist, und pA, pC, und pE die Wahrscheinlichkeitskoeffizienten für die 10-, 14- und 16,8 Å-Phasen in der Wechsellagerungsstruktur darstellen. Die obere Gleichung, berechnet mit Hilfe der Methode der kleinsten Quadrate und ohne Nebenbedingung, ergibt
On a assumé que la formation d'une structure interstratifiée dans une smectite était influencée par (1) la densité de charge de couche totale, (2) l’énergie de solvation du cation, et (3) la nature de l'agent solvant. En gardant constants les facteurs (2) et (3), il était possible de calculer les densités de charge locales moyennes $\overline {{\rm{QA}}} $ , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $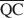 , et $\overline {{\rm{QE}}} $
, et $\overline {{\rm{QE}}} $ , qui sont nécessaires à la formation de phases mélangées 10-Å, 14-Å, et 16,8-Å dans les smectites traitées au potassium et saturées de glycol éthylène (EG). Les valeurs de $\overline {{\rm{QA}}} $
, qui sont nécessaires à la formation de phases mélangées 10-Å, 14-Å, et 16,8-Å dans les smectites traitées au potassium et saturées de glycol éthylène (EG). Les valeurs de $\overline {{\rm{QA}}} $ , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $ , et $\overline {{\rm{QE}}} $
, et $\overline {{\rm{QE}}} $ étaient respectivement 1,99, 1,2, et 0,56 esu/uc. Des smectites traitées à l'ammonium saturées de EG donnaient des densités de charge locales moyennes correspondantes de 2,7, 1,6, et 0,72 esu/uc. Les calculs ont été faits sous les conditions limitantes QA > QC > QE > 0.
étaient respectivement 1,99, 1,2, et 0,56 esu/uc. Des smectites traitées à l'ammonium saturées de EG donnaient des densités de charge locales moyennes correspondantes de 2,7, 1,6, et 0,72 esu/uc. Les calculs ont été faits sous les conditions limitantes QA > QC > QE > 0.
Pour les smectites-K saturées de EG, Qtot = 1,99pA + 1,2pC + 0,56pE, où Qtot est la charge totale (esu/uc), et pA, pC, et pE sont des coefficients de probabilité pour les phases 10-Å, 14-Å, et 16,8-Å dans la structure interstratifiée. L’équation ci-dessus calculée à l'aide des carrés moindres et sous les conditions limitantes donne