Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kopka, H
Beneke, K
and
Lagaly, G
1988.
Anionic surfactants between double metal hydroxide layers.
Journal of Colloid and Interface Science,
Vol. 123,
Issue. 2,
p.
427.
Kuma, K.
Paplawsky, W.
Gedulin, B.
and
Arrhenius, G.
1989.
Mixed-valence hydroxides as bioorganic host minerals.
Origins of life and evolution of the biosphere,
Vol. 19,
Issue. 6,
p.
573.
Thevenot, F.
Szymanski, R.
and
Chaumette, P.
1989.
Preparation and Characterization of Al-Rich Zn-Al Hydrotalcite-Like Compounds.
Clays and Clay Minerals,
Vol. 37,
Issue. 5,
p.
396.
Hansen, H. C. B.
and
Taylor, R. M.
1990.
Formation of synthetic analogues of double metal-hydroxy carbonate minerals under controlled pH conditions: I. The synthesis of pyroaurite and reevesite.
Clay Minerals,
Vol. 25,
Issue. 2,
p.
161.
Grey, Ian E.
and
Ragozzini, Roland
1991.
Formation and characterization of new magnesium aluminum hydroxycarbonates.
Journal of Solid State Chemistry,
Vol. 94,
Issue. 2,
p.
244.
Cavani, F.
Trifirò, F.
and
Vaccari, A.
1991.
Hydrotalcite-type anionic clays: Preparation, properties and applications..
Catalysis Today,
Vol. 11,
Issue. 2,
p.
173.
Hansen, H. C. B.
and
Taylor, R. M.
1991.
The use of glycerol intercalates in the exchange of CO32−with SO42−, NO3−or Cl−in pyroaurite-type compounds.
Clay Minerals,
Vol. 26,
Issue. 3,
p.
311.
Hofmeister, W.
and
Platen, H. Von
1992.
Crystal Chemistry and Atomic Order in Brucite-related Double-layer Structures.
Crystallography Reviews,
Vol. 3,
Issue. 1,
p.
3.
Misra, C.
and
Perrotta, A. J.
1992.
Composition and Properties of Synthetic Hydrotalcites.
Clays and Clay Minerals,
Vol. 40,
Issue. 2,
p.
145.
Bookin, A. S.
Cherkashin, V. I.
and
Drits, V. A.
1993.
Reinterpretation of the X-Ray Diffraction Patterns of Stichtite and Reevesite.
Clays and Clay Minerals,
Vol. 41,
Issue. 5,
p.
631.
Tsuji, Masamichi
Mao, Gang
Yoshida, Takashi
and
Tamaura, Yutaka
1993.
Hydrotalcites with an extended Al3+-substitution: Synthesis, simultaneous TG-DTA-MS study, and their CO2 adsorption behaviors.
Journal of Materials Research,
Vol. 8,
Issue. 5,
p.
1137.
del Arco, M
Martin, C
Martin, I
Rives, V
and
Trujillano, R
1993.
A FTIR spectroscopic study of surface acidity and basicity of mixed Mg, Al-oxides obtained by thermal decomposition of hydrotalcite.
Spectrochimica Acta Part A: Molecular Spectroscopy,
Vol. 49,
Issue. 11,
p.
1575.
Clause, O.
Goncalves Coelho, M.
Gazzano, M.
Matteuzzi, D.
Trifirò, F.
and
Vaccari, A.
1993.
Synthesis and thermal reactivity of nickel-containing anionic clays.
Applied Clay Science,
Vol. 8,
Issue. 2-3,
p.
169.
Mao, Gang
Tsuji, Masamichi
and
Tamaura, Yutaka
1993.
Synthesis and CO2 Adsorption Features of a Hydrotalcite-Like Compound of the Mg2+-Al3+-Fe(CN)64- System with High Layer-Charge Density.
Clays and Clay Minerals,
Vol. 41,
Issue. 6,
p.
731.
Abdelouas, A.
Crovisier, J. L.
Lutze, W.
Fritz, B.
Mosser, A.
and
Müller, R.
1994.
Formation of Hydrotalcite-like Compounds During R7T7 Nuclear Waste Glass and Basaltic Glass Alteration.
Clays and Clay Minerals,
Vol. 42,
Issue. 5,
p.
526.
Schilling, Paul J.
Roy, Amitava
and
Eaton, Harvill C.
1994.
Reply to “Comment on ‘Activation of Ground Blast‐Furnace Slag by Alkali‐Metal and Alkaline‐Earth Hydroxides’”.
Journal of the American Ceramic Society,
Vol. 77,
Issue. 4,
p.
1117.
Kooli, F.
Rives, V.
Ulibarri, M.A.
and
Jones, W.
1994.
Pillaring of Layered Double Hydroxides Possessing Variable Layer Charge with Vanadium Polyoxoanions.
MRS Proceedings,
Vol. 371,
Issue. ,
Cai, Heng
Hillier, Andrew C.
Franklin, Kevin R.
Nunn, C. Craig
and
Ward, Michael D.
1994.
Nanoscale Imaging of Molecular Adsorption.
Science,
Vol. 266,
Issue. 5190,
p.
1551.
Pitsch, S.
Eschenmoser, A.
Gedulin, B.
Hui, S.
and
Arrhenius, G.
1995.
Mineral induced formation of sugar phosphates.
Origins of life and evolution of the biosphere,
Vol. 25,
Issue. 4,
p.
297.
Kooli, F.
Kosuge, K.
and
Tsunashima, A.
1995.
Mg-Zn-Al-CO3 and Zn-Cu-Al-CO3 hydrotalcite-like compounds: Preparation and characterization.
Journal of Materials Science,
Vol. 30,
Issue. 18,
p.
4591.
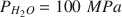 and T = 100°–350°C. Starting materials were MgO, γ-Al2O3, H2O, and MgC2O4·2H2O. The synthesis depended on temperature, pressure, the Al/(Al + Mg) ratio x, and the CO2 content of the starting material. Previously an Al content of x = 0.33 was thought to be the upper limit in these double-layer compounds, but by using pressure the Al-content was increased to x = 0.44. Up to x = 0.33, a0 decreased linearly to about 3.04 Å, but for x ≥0.33, a0 remained nearly constant at this value. For the synthesized products the layer thickness c’ varied between 7.40 and 7.57 Å in contrast to the natural phases wherein c’ varies from 7.60 to 7.80 Å. At higher temperatures CO2-free syntheses, i.e., those without Mg-oxalate, resulted in a disordered hydrotalcite-like phase. The transition temperature between the ordered and the disordered hydrotalcite-like phase depended on the Al-content, x.
and T = 100°–350°C. Starting materials were MgO, γ-Al2O3, H2O, and MgC2O4·2H2O. The synthesis depended on temperature, pressure, the Al/(Al + Mg) ratio x, and the CO2 content of the starting material. Previously an Al content of x = 0.33 was thought to be the upper limit in these double-layer compounds, but by using pressure the Al-content was increased to x = 0.44. Up to x = 0.33, a0 decreased linearly to about 3.04 Å, but for x ≥0.33, a0 remained nearly constant at this value. For the synthesized products the layer thickness c’ varied between 7.40 and 7.57 Å in contrast to the natural phases wherein c’ varies from 7.60 to 7.80 Å. At higher temperatures CO2-free syntheses, i.e., those without Mg-oxalate, resulted in a disordered hydrotalcite-like phase. The transition temperature between the ordered and the disordered hydrotalcite-like phase depended on the Al-content, x.

