Introduction
Sexual dysfunction is a prevalent symptom of major depressive disorder (MDD) as well as a side effect of treatment with serotonergic antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs). Antidepressant treatment may improve sexual dysfunction in some individuals, as sexual functioning may improve as depressive symptoms improve.Reference Gelenberg, Dunner, Rothschild, Pedersen, Dorries and Ninan1–Reference Montejo, Llorca, Izquierdo and Rico-Villademoros3 However, treatment-emergent sexual dysfunction (TESD) is a common side effect of serotonergic antidepressant drug therapy, with an estimated prevalence ranging from 4% to 73%, depending on the antidepressant administered.Reference Montejo, Llorca, Izquierdo and Rico-Villademoros3–Reference Clayton, Pradko and Croft5 This broad prevalence range may reflect the variation in TESD rates among antidepressant agents, but it may also be impacted by patient demographics and differences in assessment tools used across studies.Reference Serretti and Chiesa6
SSRIs and SNRIs affect all three phases of the sexual response cycle (desire, arousal, and orgasm).Reference Montejo, Llorca, Izquierdo and Rico-Villademoros3, Reference Clayton, Pradko and Croft5, Reference Clayton, Kornstein, Prakash, Mallinckrodt and Wohlreich7 Moreover, TESD may be seen early in treatment and persist after depressive symptoms have been moderated. Sexual dysfunction is reported as one of the most bothersome side effects of SSRI and SNRI treatment and can reduce self-esteem and quality of life and burden interpersonal relationships.Reference Ishak, Christensen and Sayer2, Reference Williams, Edin, Hogue, Fehnel and Baldwin4, Reference Clayton, Kornstein, Prakash, Mallinckrodt and Wohlreich7–Reference Duenas, Lee and Brnabic12
MDD is a recurrent and often chronic condition requiring long-term treatment. In a study of antidepressant usage in the USA in the years between 2011 and 2014, approximately 21% of men and 27% of women reported having taken antidepressant treatment for 10 or more years.Reference Pratt, Brody and Gu13 Patients with adequately treated depressive symptoms who are experiencing TESD may not mention their sexual side effects to their prescriber, which may lead to noncompliance with treatment or discontinuation of treatment altogether.Reference Montejo, Llorca, Izquierdo and Rico-Villademoros3, Reference Chokka and Hankey14
Management strategies to alleviate TESD include dosing changes, drug holidays, add-on therapies to alleviate sexual dysfunction (such as with bupropion), and switching to another antidepressant.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15–Reference Clayton, Warnock, Kornstein, Pinkerton, Sheldon-Keller and McGarvey17 Switching to a different antidepressant is common with patients who are not adequately responding to treatment; however, there is a lack of controlled studies evaluating directly switching antidepressants in patients whose depressive symptoms are adequately treated by their existing antidepressant.Reference Taylor, Rudkin, Bullemor-Day, Lubin, Chukwujekwu and Hawton18 More studies are needed to evaluate the effects of switching therapy with regard to improvement in TESD, maintenance of antidepressant efficacy, and experience of adverse events, and to identify patient-specific characteristics, such as prior SSRI treatment, age, or MDD history, impacting these outcomes.
Vortioxetine is a multimodal antidepressant for the treatment of MDD. It combines two pharmacological modes of action: direct modulation of 5-HT receptor activity and serotonin reuptake inhibition.Reference Bang-Andersen, Ruhland and Jorgensen19–21 During the vortioxetine development program, several randomized, placebo-controlled trials in adults with MDD were conducted that prospectively assessed TESD using the Arizona Sexual Experiences Scale (ASEX).Reference Baldwin, Loft and Dragheim22–Reference Mahableshwarkar, Jacobsen and Chen26 The data from these studies indicated that lower doses of vortioxetine had incidences of TESD similar to placebo, and while TESD increased with increasing dose, no vortioxetine dose had a significantly greater risk of developing TESD compared with placebo.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton27
To assess the effects of directly switching to vortioxetine in MDD patients who are experiencing TESD attributed to their current SSRI treatment (citalopram, sertraline, or paroxetine), but whose depressive symptoms are well-treated, a head-to-head trial of vortioxetine vs. escitalopram (an SSRI) was conducted. Results of the primary study have been previously published.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 The primary analyses demonstrated that vortioxetine was superior to escitalopram in improving SSRI-induced sexual dysfunction as assessed by the Changes in Sexual Functioning Questionnaire-14 (CSFQ-14), while maintaining antidepressant efficacy in adult men and women with MDD. Greater improvements were seen in the vortioxetine group compared with the escitalopram group on all five dimensions of the CSFQ-14, and were significantly superior on four of five dimensions. Vortioxetine was significantly superior to escitalopram on all three phases of the sexual functioning cycle assessed by the CSFQ-14. Although the study was not powered to detect differences between subgroups, greater improvements in sexual functioning were observed in vortioxetine-treated patients compared with escitalopram-treated patients when assessed according to sex, age, and baseline CGI-S scores.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15
The objective of the current study is to provide additional descriptive characteristics and prespecified and post-hoc analyses of TESD, antidepressant efficacy, and tolerability as a result of directly switching adults with well-treated MDD but with SSRI-induced sexual dysfunction from SSRI monotherapy to vortioxetine or escitalopram monotherapy. Prespecified analyses included CSFQ-14 individual item level analyses for the vortioxetine vs. escitalopram groups. Post-hoc analyses included the measurement of CSFQ-14 total scores for the escitalopram or vortioxetine groups based on pre-switch SSRI and sex. Additional analyses were conducted to determine the influence of baseline factors such as age, duration of prior SSRI treatment, history of MDD treatment, number of previous major depressive episodes (MDEs), and any history of childhood traumatic events on the CSFQ-14 total score after vortioxetine or escitalopram treatment. The efficacy of vortioxetine or escitalopram for the treatment of depressive symptoms was compared to pre-switch SSRI efficacy against symptoms. Finally, treatment-emergent adverse events (TEAEs) were analyzed for both vortioxetine and escitalopram according to the baseline factors mentioned above. Additional analyses regarding nausea were conducted because this was the most frequently reported TEAE in participants treated with vortioxetine in the primary study.
Methods
Study design
The primary study was an 8-week, phase IIIb, multicenter, randomized, double-blind, parallel-group, flexible-dose, head-to-head comparison of vortioxetine and escitalopram in men and women with well-treated MDD (stable depressive symptoms with Clinical Global Impressions-Severity [CGI-S] ≤3) who were experiencing SSRI-induced TESD (study NCT01364649).Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 Participants who were previously administered citalopram, sertraline, or paroxetine for at least 8 weeks before study commencement were eligible for the switch to vortioxetine or escitalopram. The objective of the primary study was to evaluate the improvement in SSRI-induced sexual dysfunction in participants switched to vortioxetine vs. escitalopram as assessed by the CSFQ-14. In the clinical study, participants were randomized equally (1:1) to receive flexible doses of either vortioxetine (10 and 20 mg) or escitalopram (10 and 20 mg) once daily.
The study was conducted according to the World Medical Association Declaration of Helsinki, ICH Guidelines, US Code of Federal Regulations for Good Clinical Practice, and all applicable federal, regional, and local regulatory requirements. An ethics committee approved the protocol and written informed consent was obtained from each participant before any procedures were performed. The trial was conducted from June 2011 to December 2013 and is registered with ClinicalTrials.gov (NCT1364649). Complete details of the study design and treatment regimen have been previously described.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15
Participants
Men and women aged 18–55 years were included in the study if they were receiving a stable regimen of citalopram, paroxetine, or sertraline monotherapy (immediate pre-switch treatment) for ≥8 weeks to treat an MDE. Diagnosis of MDE was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. This age range was chosen because rates of primary sexual dysfunction, as well as comorbid conditions or treatments associated with TESD are known to increase with age.
Eligible participants had stable depression symptoms as judged by the investigator and indicated by CGI-S scores ≤3 at screening and baseline visits.Reference Guy28 In addition, all eligible participants had to have been sexually active at least every 2 weeks before onset of the current MDE and/or SSRI use; experienced TESD (assessed by CSFQ-14 total scores of ≤41 [women] or ≤47 [men]) that was attributed to their current SSRI treatment per investigator judgment; had stable CGI-S scores of ≤3 at screening and baseline; and been suitable for a switch in treatment.
Participants were excluded if their sexual dysfunction was associated with an etiology other than SSRI treatment, if they had experienced a major relationship change during SSRI treatment, or if they or their sexual partners were planning to initiate treatment for sexual dysfunction during the course of the study.
Treatments
In the clinical study, participants were randomized at baseline (week 0) to receive 10 mg escitalopram or vortioxetine for the first week, and then were titrated up to 20 mg for the second week. After the second week flexible dosing (10/20 mg) was permitted at scheduled study visits.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15
Herein, we describe additional analyses conducted to further evaluate the effects of participant characteristics such as depression history, prior SSRI treatment, sex, and age (in women), on improvements in sexual functioning observed after the switch to vortioxetine or escitalopram.
Outcome measures
Sexual functioning was assessed at each clinic visit using the CSFQ-14, a validated, structured, self-reported questionnaire.Reference Keller, McGarvey and Clayton29 The questionnaire includes 14 items that are scored on a 5-point Likert scale. There are separate versions for men and women. Possible total scores range from 14 to 70, where lower scores indicate worsened/poorer sexual functioning.Reference Keller, McGarvey and Clayton29 Sexual functioning outcomes were assessed using CSFQ-14 change in total scores from baseline to week 8. Additional outcomes included antidepressant efficacy as assessed by the Montgomery–Åsberg Depression Rating Scale (MADRS), CGI-S, and CGI-improvement (CGI-I) scales.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 TEAEs were assessed for all participants at baseline and at the end of weeks 1, 2, 4, 6, and 8.
Statistical analysis
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina, USA). The full analysis set (FAS) included all participants who were randomized, received ≥1 dose of a study drug, and had at least one valid post-baseline value for assessment of CSFQ-14 total score.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 Descriptive statistics were reported for TEAEs for all participants who were randomized and received ≥1 dose of double-blind study medication. The primary study was not powered to detect significant differences between the subgroups we are reporting on in this post-hoc analysis. Therefore, more attention should be paid to the magnitude of differences if any. Differences are not significant unless identified as such.
Prespecified analyses of changes in CSFQ-14 individual item scores were analyzed for the vortioxetine vs. escitalopram groups after 8 weeks of treatment. The post-hoc analyses of CSFQ-14 changes in total scores by prior SSRI treatment were conducted based on the following subgroups separately: age, duration of treatment with prior SSRI, previous SSRI, number of previous MDEs, history of MDD treatment, history of childhood traumatic events (including sexual), and MADRS remission status (MADRS total score ≤10). The age cutoff selected for women was related to reduced hormone levels in perimenopausal women, which could impact sexual functioning.Reference Clayton and Hamilton30 Thus, the cohort of women was divided into participants younger or older than 45 years of age. Administration of antidepressants for more than 1 year is typically associated with long-term treatment. Therefore, previous SSRI usage was divided into two subgroups: participants taking SSRIs for ≤1 year or >1 year. Similarly, the number of prior MDEs can influence recurrence and response to treatment.Reference Bulloch, Williams, Lavorato and Patten31 The first MDEs often respond differently to treatment and more than three MDEs could be difficult to treat. To address this, MDE subgroups were separated based on number of occurrences.
Change from baseline in CSFQ-14 total score was analyzed for each of the subgroups using observed data based on a mixed model for repeated measurements (MMRM) with treatment, center, week, and treatment-by-week interaction as fixed effects, baseline CSFQ-14 total score-by-week as a covariate, and a completely unstructured covariance matrix. Similar approaches were applied to other continuous efficacy endpoints such as CSFQ-14, individual item scores and other subscale scores, MADRS total score, CGI-S, and CGI-I scores. MADRS remission rates were analyzed for each group by logistic regression adjusting for baseline score and treatment using the last observation carried forward method. All statistical tests were two-sided with a 0.05 significance level.
Results
Participant disposition
Of the 711 participants screened, 447 were randomized and 348 (77.9%) completed the study. Fifty-six (24.9%) participants in the vortioxetine group and 43 (19.4%) participants in the escitalopram group discontinued the study prematurely, with withdrawal due to a TEAE occurring in numerically more vortioxetine-treated participants (n = 20; 8.9%) than escitalopram-treated participants (n = 14; 6.3%).Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 At baseline, most participants were in remission (MADRS total score ≤10; 77.3% [escitalopram] and 78.7% [vortioxetine] with remission defined as a MADRS total score ≤10). Mean MADRS scores at baseline were 7.9 and 8.3 for vortioxetine and escitalopram, respectively. Mean CSFQ-14 scores at baseline for vortioxetine and escitalopram were 36.5 and 36.3, respectively, indicating significant sexual dysfunction. After 2 weeks of treatment, the majority of participants received the 20-mg dose of therapy (escitalopram, 71.9%; vortioxetine, 65.6%). No significant differences in baseline demographic or clinical characteristics were observed between vortioxetine or escitalopram treatment groups in the primary study.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15
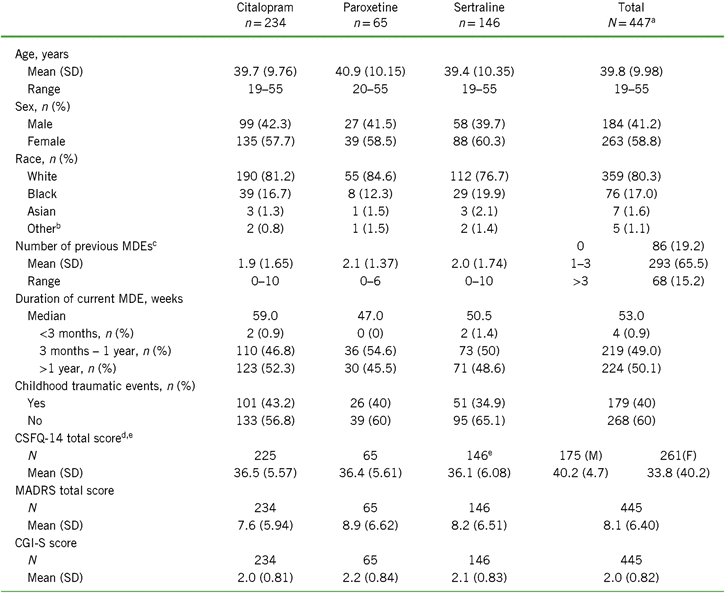
Of the 447 randomized participants, about half were previously treated with citalopram (n = 235), followed by sertraline (n = 146) and paroxetine (n = 66). Because of the small sample size in the paroxetine group, conclusions should be made with caution. Baseline CSFQ-14 total scores and mean age were similar across prior SSRI treatments (Table 1). Over 65% of all participants indicated having one to three prior MDEs, with the remainder split approximately equally between zero and more than three prior MDEs. Participants who were switched from citalopram had the lowest number of prior MDEs ([mean ± standard deviation (SD)] 1.9 ± 1.65 vs. 2.1 ± 1.37 for paroxetine and 2.0 ± 1.74 for sertraline), the lowest mean MADRS score (7.6 ± 5.94 vs. 8.9 ± 6.62 and 8.5 ± 6.97 for paroxetine and sertraline, respectively), and the lowest mean CGI-S score (2.0 ± 0.81 vs. 2.2 ± 0.84 and 2.1 ± 0.83 for paroxetine and sertraline, respectively) (Table 1). Conversely, the paroxetine subgroup had the highest number of prior MDEs, highest mean MADRS score, and mean CGI-S score. The sertraline group had the highest percentage of blacks and the lowest percentage of whites. Subgroup distributions were similar for duration of current MDE, with about half the participants reporting a duration between 3 months and 1 year and the other half over 1 year; the median MDE duration for all subgroups combined was 53 weeks. Overall, 40% of the participants indicated that they had childhood traumatic events, and the incidence of events was highest in the citalopram group and lowest in the sertraline group.
TABLE 1. Demographics and baseline characteristics for the total population and by SSRI received before randomization (all randomized set)
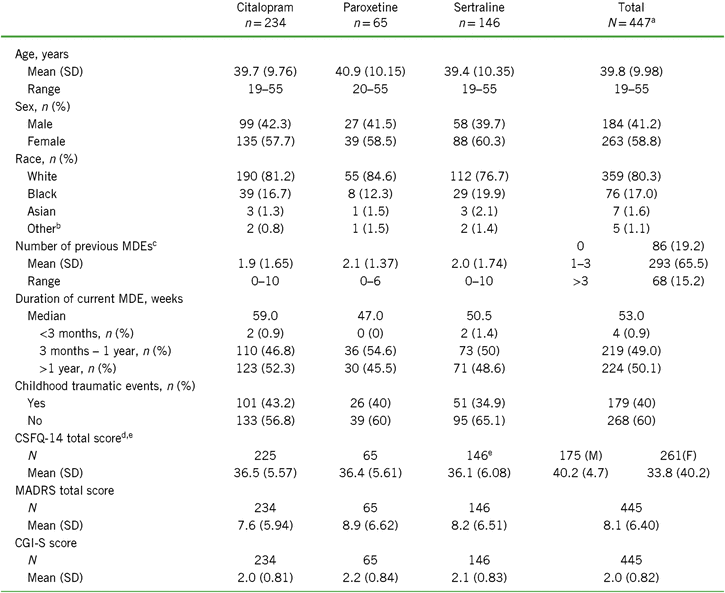
Notes: CGI-S = Clinical Global Impression–Severity of Illness scale; CSFQ-14 = Changes in Sexual Functioning Questionnaire short form; F = female; M = male; MADRS = Montgomery–Åsberg Depression Rating Scale; MDE = major depressive episode; SD = standard deviation; SSRI = selective serotonin reuptake inhibitor.
a Includes two participants who were initially randomized but did not receive study drug and were therefore not included in the SSRI subgroup analysis sets.
b Other includes American Indian, Alaska Native, Native Hawaiian, or Other Pacific Islander.
c Reported as n (%) for each range in total population.
d Total n (%) reported for men (left) and women (right).
e Some participants have missing baseline efficacy values.
Change from baseline in CSFQ-14 in vortioxetine vs. escitalopram participants
As reported in Jacobsen et al., participants who were switched to vortioxetine had significantly greater CSFQ-14 total score improvements throughout the 8 weeks of treatment than those switched to escitalopram, with differences between the two groups reaching statistical significance at weeks 4 and 8.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 For this report, prespecified analyses included CSFQ-14 individual item level analyses for the vortioxetine vs. escitalopram groups. These analyses revealed that improvements were greater in the vortioxetine group than the escitalopram group in nearly all measured individual items of sexual functioning (Figure 1).
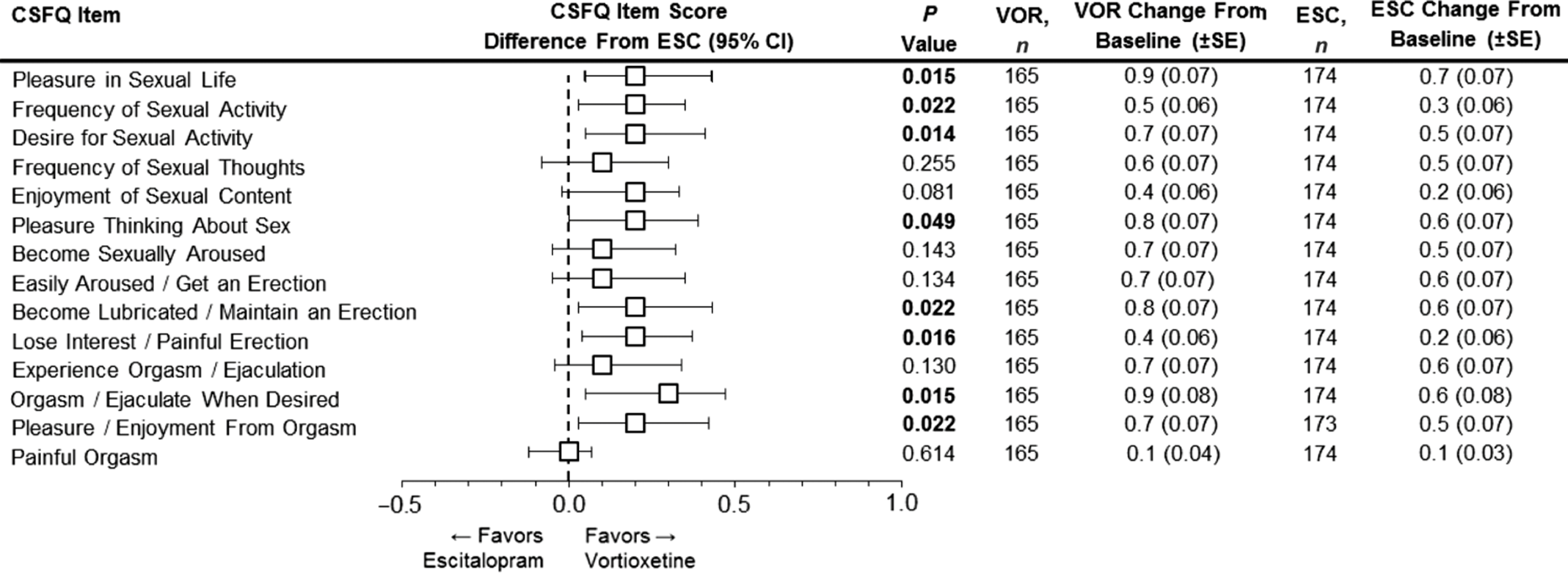
FIGURE 1. Analysis of the change from baseline in CSFQ-14 individual item scores at week 8. Forest plot shows the LS mean difference between vortioxetine and escitalopram in relation to antidepressant switching and sexual function as measured by the CSFQ-14. Individual CSFQ-14 items, which together measure the 5 dimensions and 3 phases of sexual functioning (MMRM, FAS), are shown. Changes from baseline (LS mean) for the CSFQ-14 individual items scores at week 8 for both vortioxetine and escitalopram are shown to the right. Bold P-values indicate statistical significance. CI = confidence interval; CSFQ-14 = Changes in Sexual Functioning Questionnaire-14; ESC = escitalopram; FAS = full analysis set; LS mean = least-squares mean; MMRM = mixed model for repeated measurements; SE = standard error; VOR = vortioxetine.
Change from baseline to week 8 in CSFQ-14 total scores by pre-switch SSRI
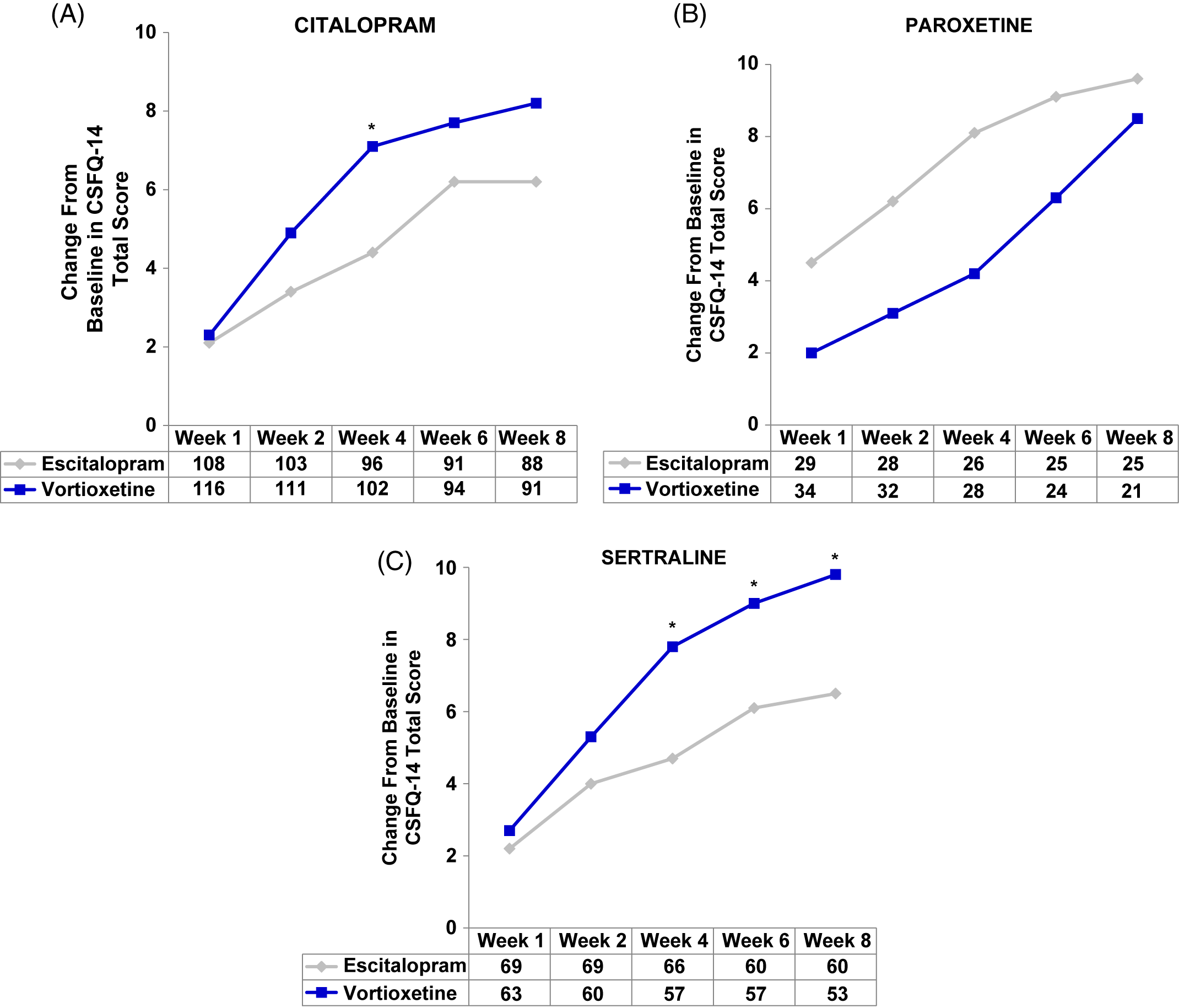
In the post-hoc analyses, improvements in sexual function were observed with both vortioxetine and escitalopram regardless of prior SSRI (herein written as: prior SSRI/switch therapy). Participants in the citalopram/vortioxetine and sertraline/vortioxetine subgroups experienced a greater improvement in overall sexual functioning than did participants who were treated with citalopram/escitalopram or sertraline/escitalopram, respectively, while the greatest improvement with escitalopram was demonstrated in the smallest subgroup: paroxetine/escitalopram (Figure 2A–C and Table 2). Clinically meaningful improvements (increase of two or more points on CSFQ-14) were seen as early as week 2 (Figure 2A–C). Improvements in sexual functioning continued to increase for those treated with vortioxetine, while participants treated with escitalopram appeared to experience a plateau.
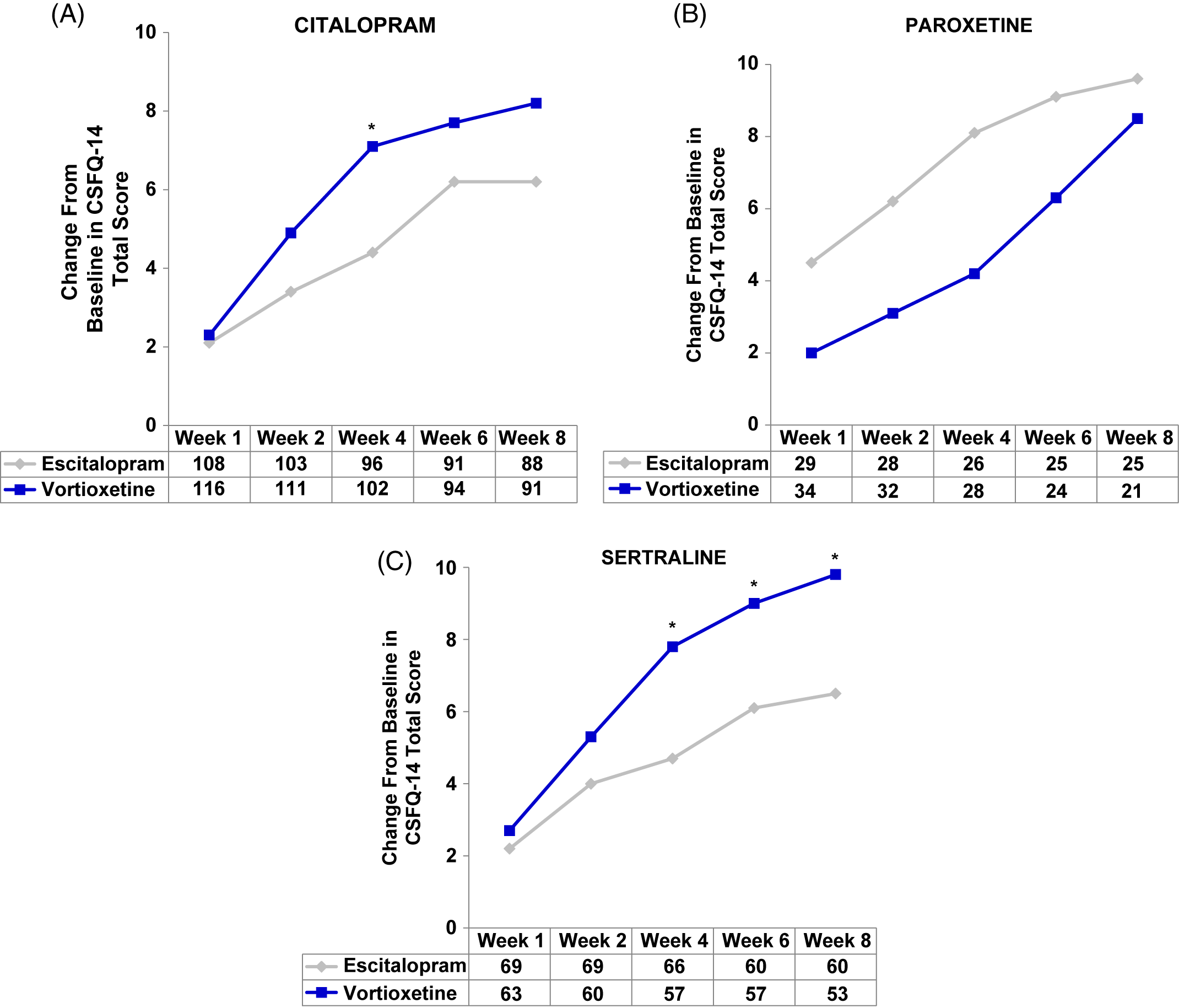
FIGURE 2. (A–C) Analysis of change from baseline in CSFQ-14 total score by SSRIs received before randomization, i.e., pre-switch (MMRM, FAS). Changes from baseline in CSFQ-14 total scores were assessed at weeks 1, 2, 4, 6, and 8 for (A) citalopram, (B) paroxetine, and (C) sertraline subgroups. The tables below each graph show the number of patients analyzed at each time point in the vortioxetine and escitalopram treatment groups. P-values shown are for the differences between treatment groups. CIT = citalopram; CSFQ-14 = Changes in Sexual Functioning Questionnaire–Short Form; ESC = escitalopram; PAR = paroxetine; SER = sertraline; VOR = vortioxetine; wk = week; *P<0.05 vs escitalopram.
TABLE 2. Change from baseline to week 8 in CSFQ-14 total score based on treatment cohort and previous SSRI
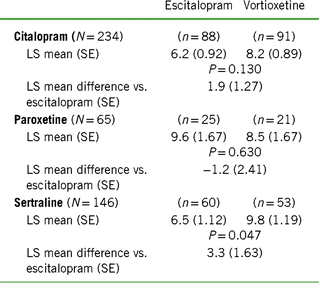
Notes: LS mean = least-squares mean; SE = standard error.
Values for “n” are based on the number of participants at week 8.
Positive scores indicate greater improvement for vortioxetine.
We next evaluated the effect of the participant’s sex on CSFQ-14 improvements across SSRI subgroups (Table 3 and Figure 3). Following 8 weeks of treatment, improvements were seen across all SSRI subgroups for both sexes. For men switching from citalopram, greater improvements in sexual functioning were seen with vortioxetine vs. escitalopram; for women, the degree of improvement was similar between those treated with citalopram/vortioxetine or citalopram/escitalopram. For women switching from sertraline, a greater improvement was observed with vortioxetine than with escitalopram, with a similar, albeit lesser degree, of improvement observed for men. Greater improvements were seen in men switching from paroxetine to escitalopram (paroxetine/escitalopram) than to vortioxetine (paroxetine/vortioxetine), although observations from the paroxetine subgroup should be interpreted with caution due to the small sample size. Overall, for men, the greatest improvements in sexual functioning were observed in the citalopram/vortioxetine group, while the greatest improvement for women were those seen in the sertraline/vortioxetine group.
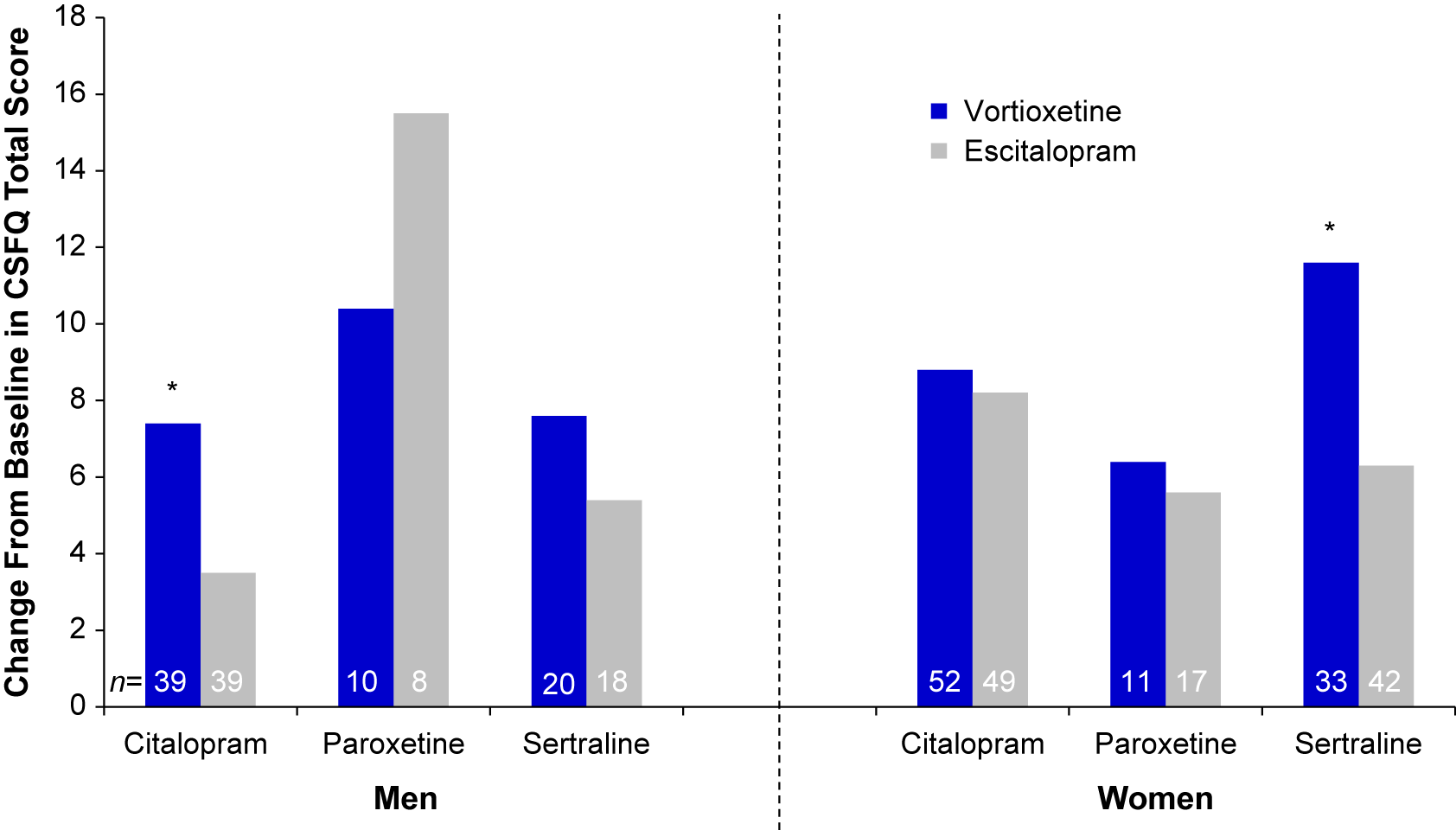
FIGURE 3. Mean change from baseline through week 8 in CSFQ-14 total score by sex and prior SSRI (FAS, MMRM, LS means). *P<0.05 vs. escitalopram. CSFQ-14 = Changes in Sexual Functioning Questionnaire–Short Form; FAS = full analysis set; LS mean = least-squares mean; MMRM = mixed model for repeated measurements; SSRI = selective serotonin reuptake inhibitors.
Table 3. Change from baseline to week 8 in CSFQ-14 total score based on sex according to treatment group and prior SSRI*
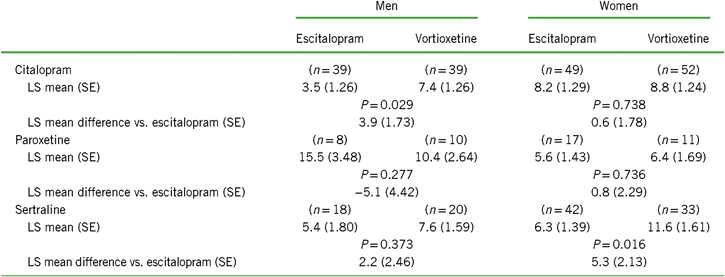
Notes: LS mean = least-squares mean; SE = standard error.
Values for “n” are based on the number of participants at week 8.
Positive scores indicate greater improvement for vortioxetine.
Effects of participant demographics and history on post-switch CSFQ-14 total scores
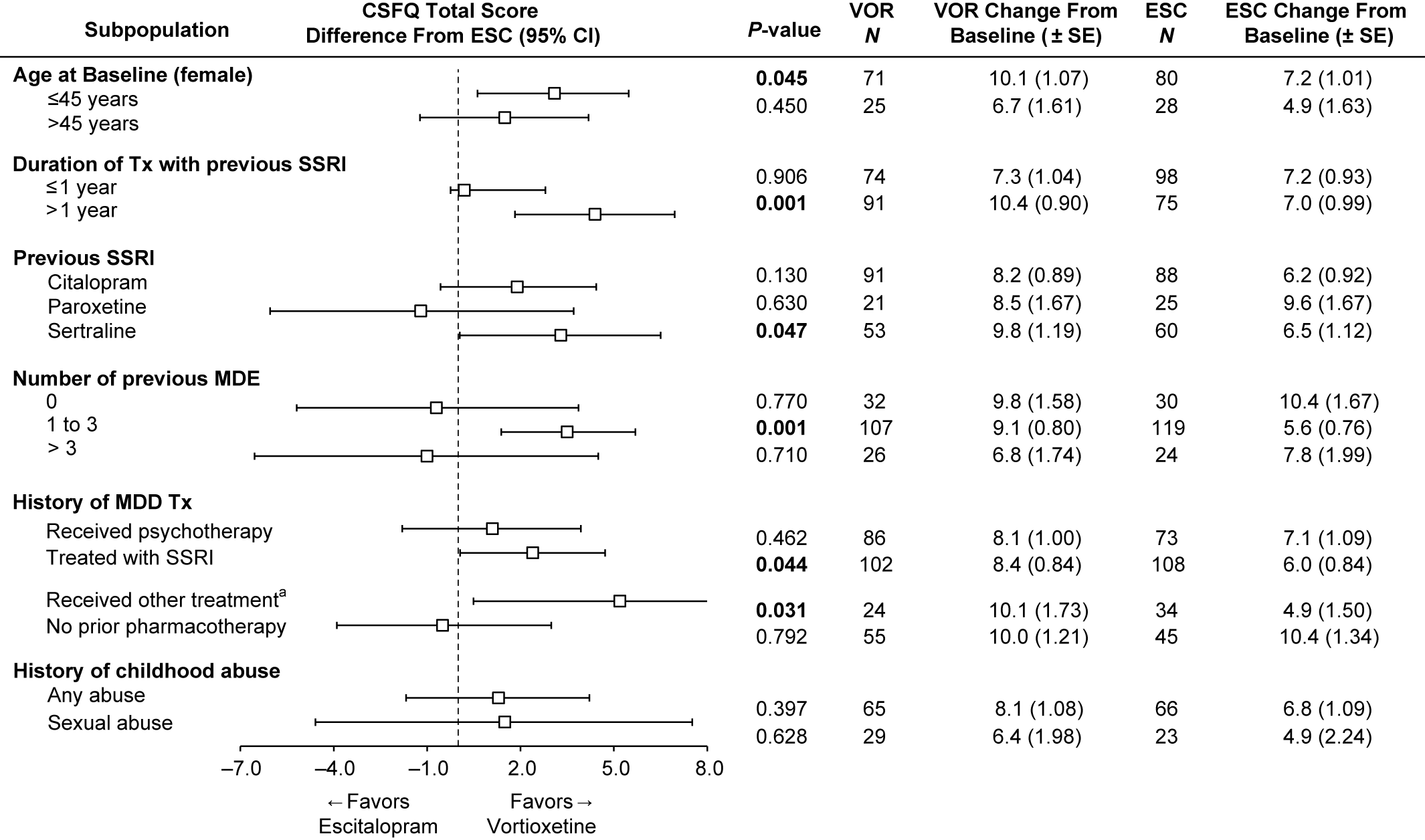
To investigate the effects of participant characteristics on post-switch sexual functioning, we examined the change from baseline in CSFQ-14 total scores at week 8 in subpopulations by age (≤45 or >45 years, female only), duration of treatment with prior SSRI (≤1 or >1 year), number of previous MDEs (0, 1–3, or >3), history of MDD treatments (psychotherapy, SSRI, SNRI, other, no prior pharmacotherapy), and childhood traumatic events (any or sexual) (Figure 4). The analysis by prior SSRI treatment is included in Figure 4 as well.
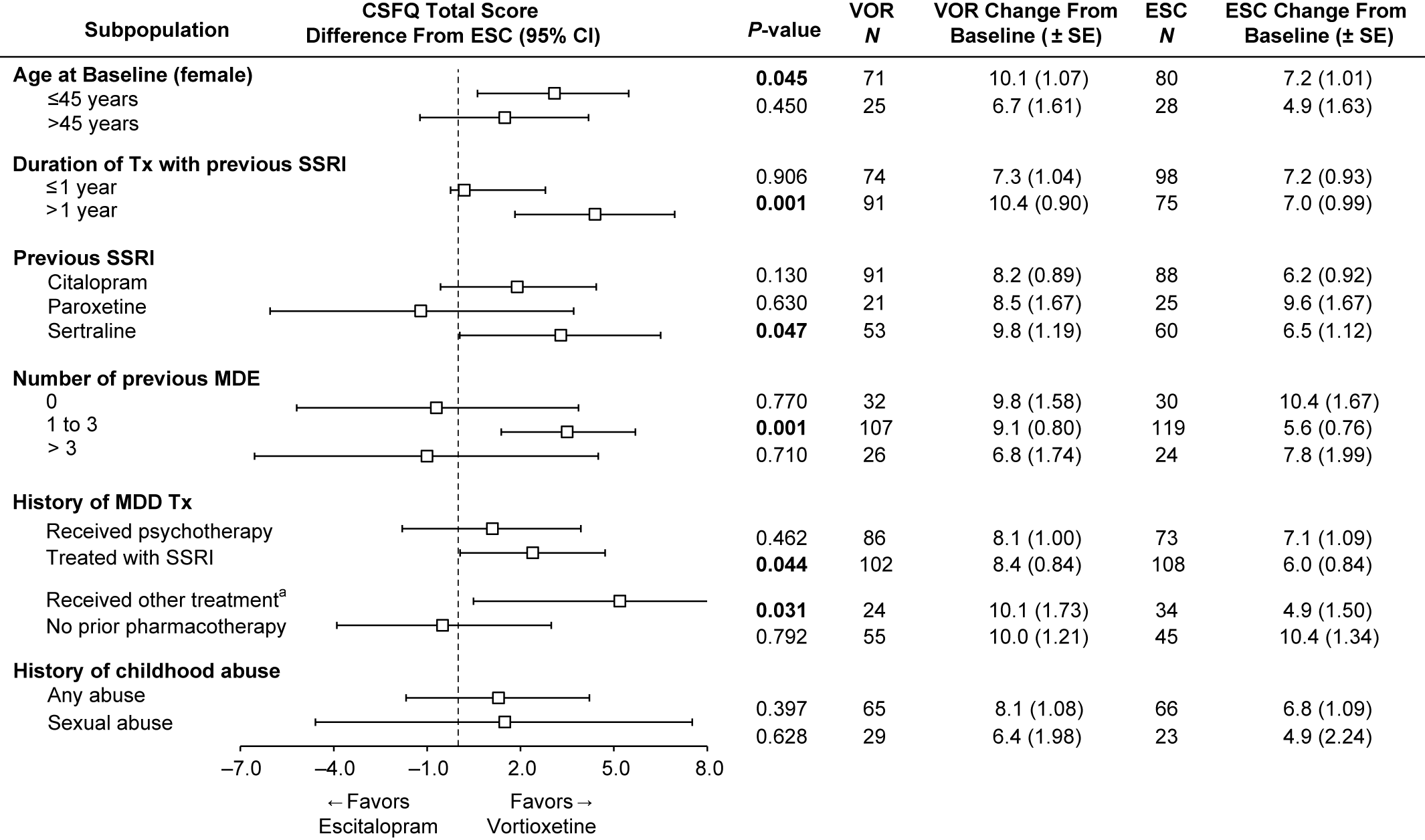
FIGURE 4. Change from baseline in CSFQ-14 total score at week 8 and impact of treatment according to subgroups (FAS, MMRM, LS means). Bolded P-value indicates a statistically significant difference between vortioxetine and escitalopram. aIncludes other antidepressant medications. ESC = escitalopram; FAS = full analysis set; LS mean = least-squares mean; MDE = major depressive episode; MMRM = mixed model for repeated measurements; VOR = vortioxetine.
Our post-hoc analysis of age in the female subgroup revealed that improvement in sexual function was greater in the vortioxetine group, regardless of age. In women ≤45 years, the improvement in the vortioxetine group over the escitalopram group was also statistically significant (mean difference vs. escitalopram 2.9; P = 0.045).
Next, we investigated the effects of the duration of prior treatment with antidepressants and the number of prior MDEs on sexual functioning after switching (Figure 4). In the subgroup of participants with ≤1 year of prior SSRI treatment, improvement in sexual function was similar for both vortioxetine and escitalopram groups. Interestingly, in the subgroup with >1 year of prior SSRI treatment, the improvement in participants treated with vortioxetine over escitalopram was significantly greater, with a 4.4-point difference, (95% CI: 1.83–6.96; P = 0.001). In participants with a history of no prior MDE (first episode), the improvement in sexual functioning from baseline to week 8 was similar for both vortioxetine and escitalopram groups, and was higher than the other two groups (1–3, >3 MDEs). In the smallest subgroup of greater than or equal to 3 MDEs, improvement in the escitalopram group was numerically better than in the vortioxetine group, but was not significant (diff = −1.0; P = 0.710). However, in participants with a history of one to three MDEs, improvements in the vortioxetine group were significantly greater, with a difference of 3.5 (P = 0.001) vs. the escitalopram group (95% CI: 1.38–5.69; P = 0.001).
Participants with a history of prior SSRI treatment for MDEs had significantly greater improvements on vortioxetine than escitalopram (diff, P = 0.044). Participants who received “other therapies (including SNRIs)” for past treatment of MDEs also had significantly greater improvements on vortioxetine, but the N for this subgroup was low, so any conclusions should be made with caution (95% CI: 0.49–9.82; P = 0.031). Although there was a numerically greater improvement in the vortioxetine-treated group vs. the escitalopram group for participants with childhood traumatic events (any event or sexual event), the differences were not significant (Figure 4).
Antidepressant efficacy across SSRI subgroups
Participants entering the study were well-treated for depressive symptoms and had overall low mean MADRS scores and CGI-S scores at entry (Table 1). Participants who switched to vortioxetine and escitalopram maintained the antidepressant efficacy achieved with their prior SSRI as measured by MADRS and the CGI scales.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15
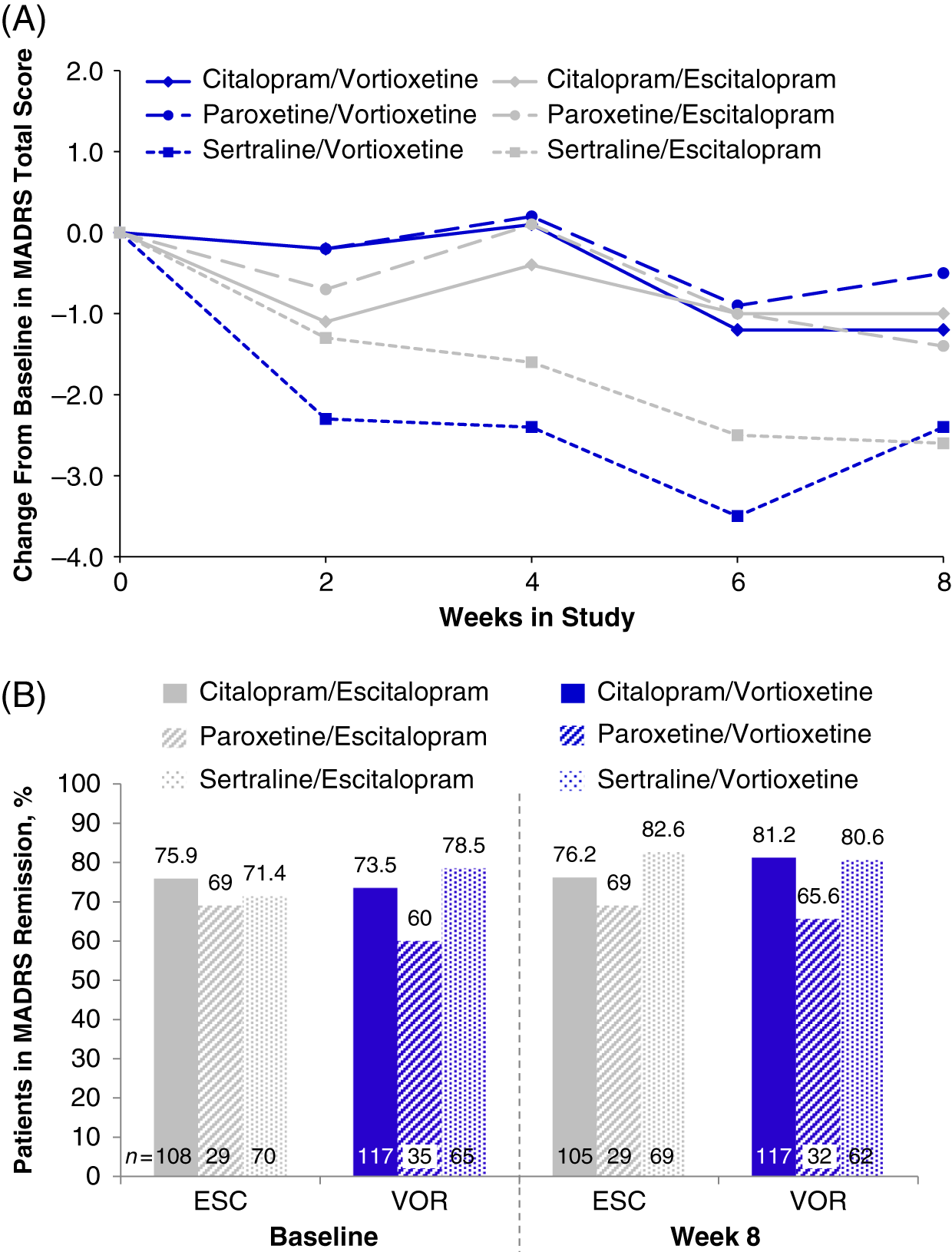
In the randomized population (n = 447), the total MADRS scores at baseline varied slightly across prior SSRI subgroups: citalopram (7.7 ± 5.92, n = 234), paroxetine (8.9 ± 6.67, n = 65), and sertraline (8.2 ± 6.51, n = 146) (Table 1). Slight improvements were seen in all prior SSRI subgroups from baseline to week 8, regardless of treatment with vortioxetine or escitalopram. The greatest mean reductions in MADRS total scores was observed in both treatment groups for participants who switched from sertraline (sertraline/vortioxetine: −2.4; sertraline/escitalopram: −2.6) (Figure 5A and Table 4). Reductions in MADRS scores were smallest for the paroxetine subgroups, and those in the paroxetine/vortioxetine subgroup had the highest mean MADRS total scores at baseline.
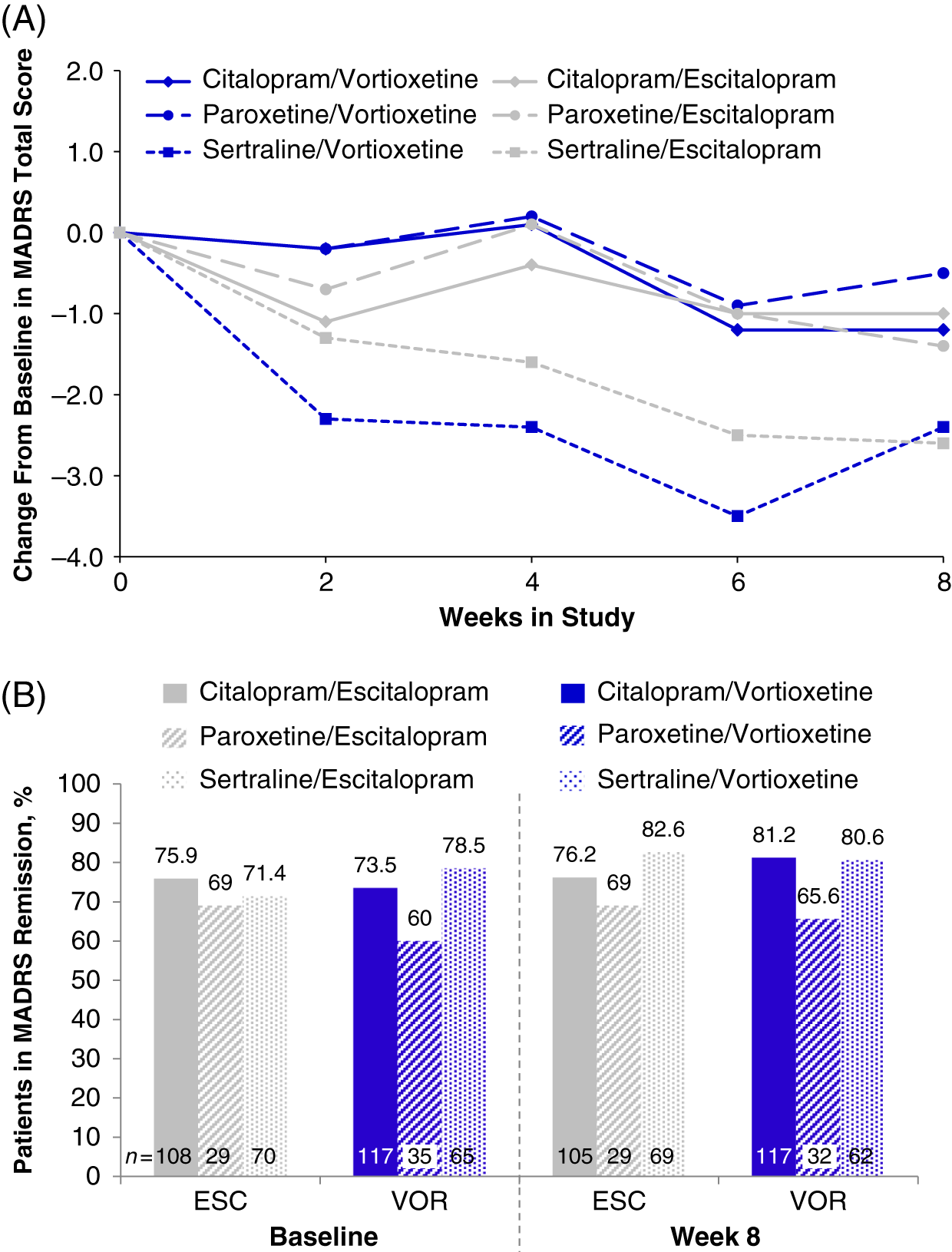
FIGURE 5. (A) Change from baseline through week 8 in MADRS total score by study visit and SSRI received before randomization, i.e., pre-switch (MMRM, LS means); week 8 comparison between vortioxetine and escitalopram by subgroup: citalopram, P = 0.832; paroxetine, P = 0.590; sertraline, P = 0.916 (B) Patients in MADRS remission (MADRS ≤10) at baseline and at week 8, analyzed by SSRI received before randomization, i.e., pre-switch (LOCF); week 8 comparison between vortioxetine and escitalopram by subgroup: citalopram, P = 0.394; paroxetine, P = 0.877; sertraline P = 0.435: LOCF = last observation carried forward; LS mean = least-squares mean; MADRS = Montgomery–Åsberg Depression Rating Scale; MMRM = mixed model for repeated measurements.
TABLE 4. Change from baseline to week 8 additional endpoints by SSRI received before randomization (FAS, OC, MMRM)
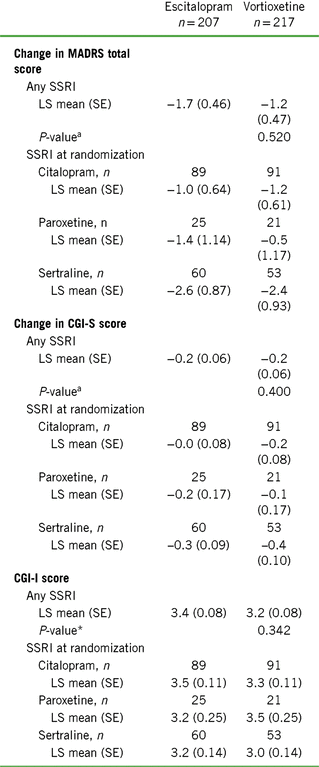
Notes: CGI-I = Clinical Global Impression–Improvement scale; CGI-S = Clinical Global Impression–Severity of Illness scale; CSFQ-14 = Changes in Sexual Functioning Questionnaire–Short Form; LS = least squares; MADRS = Montgomery–Åsberg Depression Rating Scale; SE = standard error; SSRI = selective serotonin reuptake inhibitor.
a Vortioxetine vs. escitalopram (prespecified analysis).
Changes in CGI-S scores from baseline to week 8 were small and generally consistent with the changes in MADRS total scores. CGI-I scores at week 8 were similar between treatment cohorts analyzed by prior SSRI treatment, with mean scores indicating minimal (3) to no (4) improvement. Participants in the citalopram/vortioxetine subgroup had a mean change in CGI-I score (3.3 ± 0.11) comparable to that observed for participants in the citalopram/escitalopram (3.5 ± 0.11, LS mean, SE) subgroup. A similar effect was observed between the paroxetine/vortioxetine (3.5 ± 0.25) and paroxetine/escitalopram (3.2 ± 0.25) subgroups, as well as the sertraline/vortioxetine (3.0 ± 0.14) and sertraline/escitalopram (3.2 ± 0.14) subgroups.
MADRS remission by prior SSRI treatment
At baseline, most participants were in remission (MADRS total score ≤10; 77.3% [escitalopram] and 78.7% [vortioxetine]; P = 0.902).Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 Compared with the citalopram and sertraline subgroups, the paroxetine subgroup had a lower percentage of participants in remission at week 8 (Figure 5B). In general, remission rates across all subgroups were similar throughout the 8-week treatment period (data for weeks 2, 4, and 6 are not shown). By week 8, no significant differences in the percentage of MDD remitters were observed between vortioxetine and escitalopram treatments, respectively, regardless of whether participants switched from citalopram (81.2% vs. 76.2%; P = 0.394), paroxetine (65.6% vs. 69.0%; P = 0.877), or sertraline (80.6% vs. 82.6%; P = 0.435). These data suggest that escitalopram or vortioxetine at the dosage levels administered in this study were adequate to sustain remission for at least a period of 8 weeks in participants switching from SSRI therapy.
Tolerability
Both escitalopram and vortioxetine were well tolerated, with low rates of withdrawal due to a TEAE (6.3% and 9.4%, respectively) (Table 5). Serious adverse events occurred in one participant randomized to escitalopram and in three who were randomized to vortioxetine. The most common TEAEs (incidence ≥5%) in the vortioxetine treatment group were nausea (25.0%), headache (9.4%), dizziness (8.0%), and generalized pruritus (5.8%) (Table 5). Prior SSRI treatment did not appear to influence the overall incidence or severity profile of TEAEs in either group, except for nausea. Among participants treated with vortioxetine, the incidence of treatment-emergent nausea was greatest in those previously treated with citalopram (29.2%), and lowest in those previously treated with paroxetine (20.0%) and with sertraline (20.0%) (Table 5).
TABLE 5. Summary of treatment-emergent adverse events (TEAEs) after 8 weeks of treatment in the total population and stratified by previous SSRI treatment (safety set)
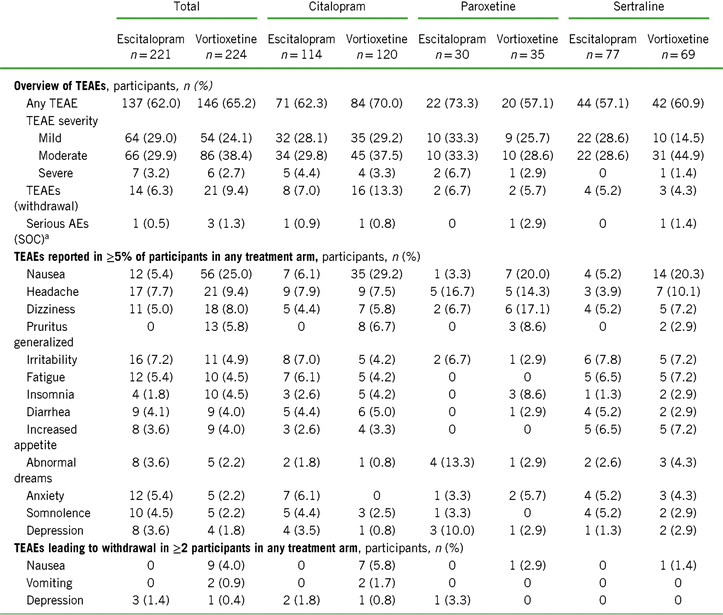
Notes: TEAE = treatment-emergent adverse event; SOC = system organ class.
a Serious AEs were mesenteric vein thrombosis, nephrolithiasis, angina pectoris, depression, and anxiety.
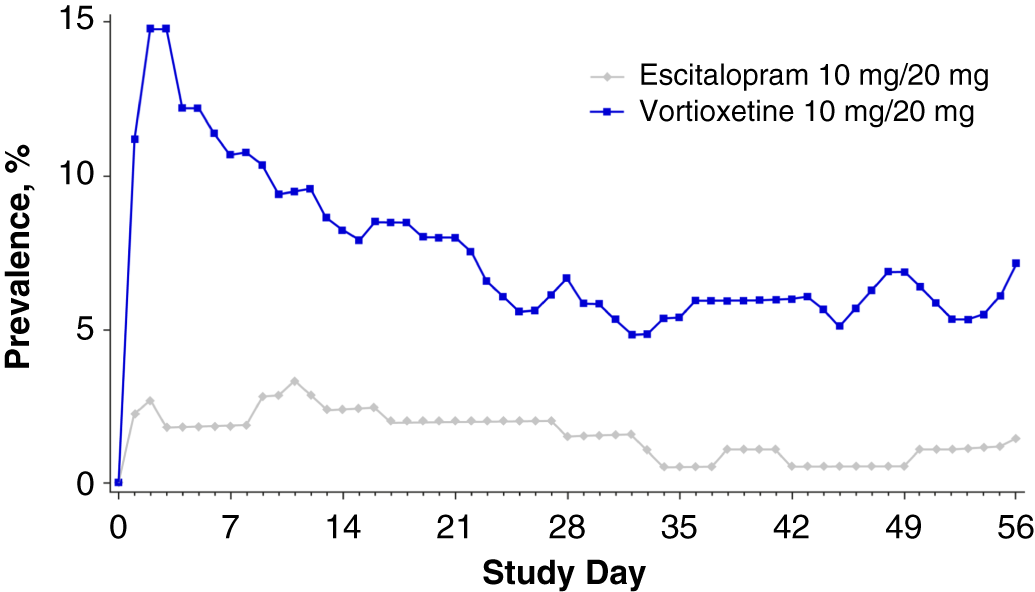
Overall, the nausea rates seen in all prior SSRI treatment groups switched to escitalopram were very low (3.3–6.1%). Nausea rates for participants switching to vortioxetine from a previous SSRI were higher (20–29.2%). Treatment discontinuations due to nausea were only reported for those participants who switched to vortioxetine (seven who switched from citalopram [5.8%] and one each from paroxetine [2.9%] or sertraline [1.4%]) (Table 5). In participants who previously took citalopram, the prevalence of nausea was higher in the vortioxetine treatment group than in the escitalopram group during all points of the treatment period (Figure 1A in Supplementary Material). This effect was also observed in participants previously treated with paroxetine or sertraline, except at various time points during week 5 (days 29–32, paroxetine; days 32–33, sertraline) (Figures 1B and 1C in Supplementary Material) and week 8 (days 52–56, sertraline) (Figure 1C in Supplementary Material).
Nausea generally occurred within the first week of treatment and resolved within 14 days of onset (Figure 6). The time-course of nausea events was similar with both escitalopram and vortioxetine, with onset most common during the first week of treatment (mean, 7.2 and 6.1 days, respectively) and duration approximately 2 weeks (mean, 14.0 and 14.2 days, respectively) (Figure 7).
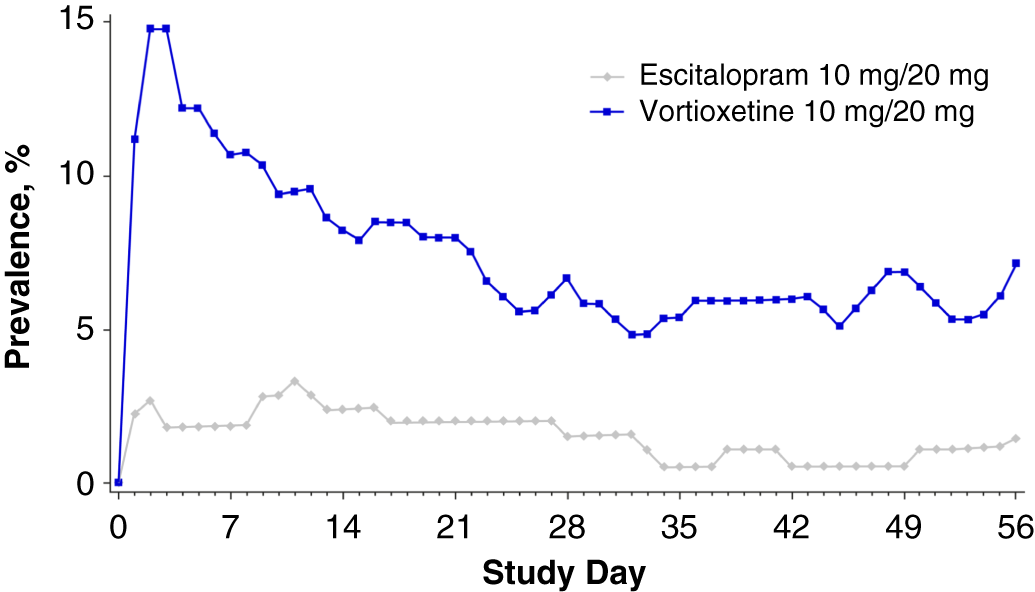
FIGURE 6. Plot of point prevalence of nausea during the treatment period (safety set).
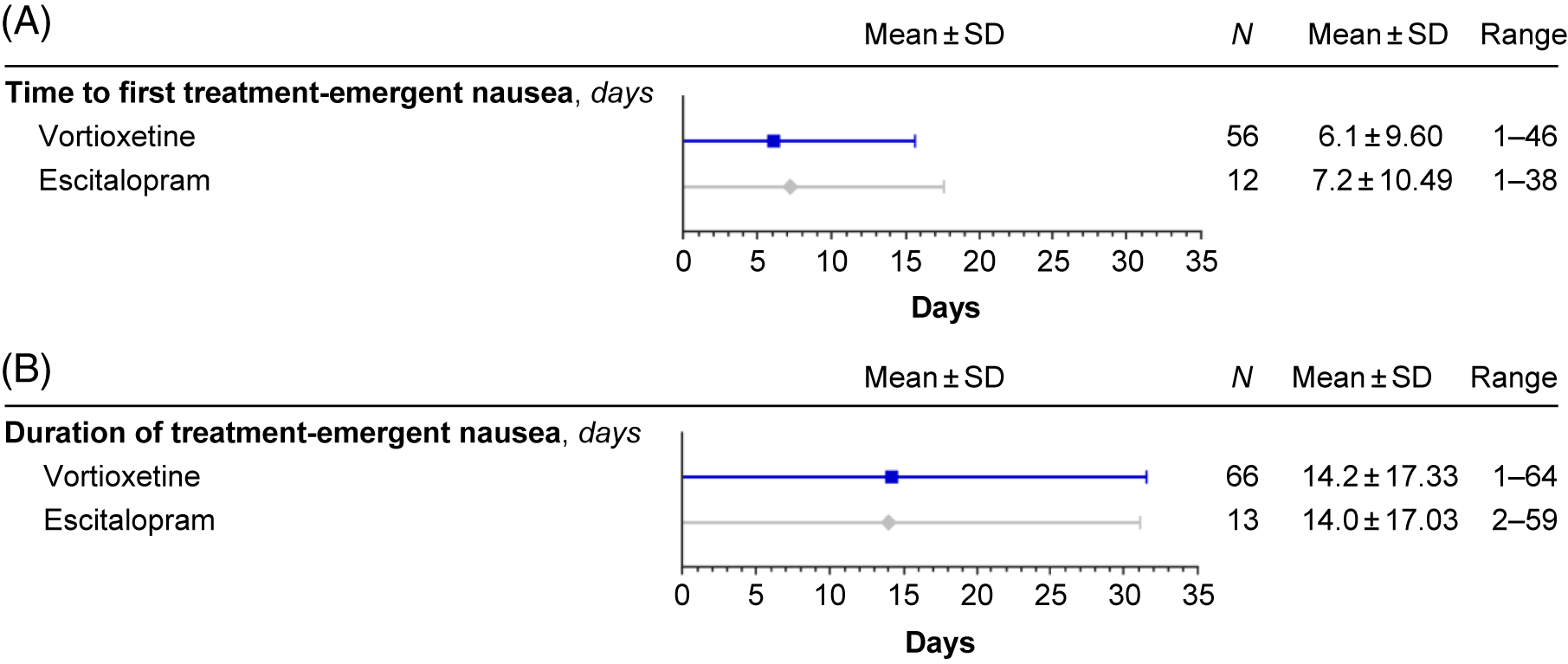
FIGURE 7. Treatment-emergent nausea: time to first (A) and duration (B) (safety set).
Discussion
Individuals with MDD whose symptoms of depression are adequately treated with SSRI therapy may experience TESD, which may negatively impact relationships and quality of life. There are few controlled studies evaluating the effects of directly switching antidepressants in patients whose depressive symptoms are adequately treated by their existing antidepressant, but who are experiencing TESD. This study provides relevant information on tolerability and efficacy of switching from SSRIs citalopram, sertraline, or paroxetine to vortioxetine or escitalopram. Considering that rates of TESD vary by antidepressant treatment, Reference Clayton, Pradko and Croft5, Reference Serretti and Chiesa6 we investigated whether the pre-switch SSRI had an effect on the post-switch TESD and efficacy with vortioxetine or escitalopram.
Regardless of prior SSRI, participants in both treatment groups demonstrated maintenance or improvement in sexual functioning following the direct switch.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 However, those who switched from sertraline to vortioxetine exhibited greater improvements in CSFQ-14 total scores than those who switched to escitalopram. Differences between treatments were not statistically significantly different for participants switching from paroxetine or citalopram. These preliminary findings suggest that the mechanisms underlying sexual side effects may respond differentially to subsequent medication management.
We also investigated whether the immediate pre-switch SSRI would affect the post-switch TESD response differently between the sexes. In men switching from paroxetine, numerically greater improvements were observed with escitalopram compared with vortioxetine, but the sample size was small and the difference was not statistically significant. Although some difference was observed between men and women in the change from baseline in CSFQ-14 function, the small number of participants in each subgroup limited the interpretation of these findings.
Because the factors that predict TESD outcomes following antidepressant switch have not been well characterized, we assessed whether participant characteristics were predictive of improvement in TESD after switching to vortioxetine vs. escitalopram. Participant characteristics including age and sex (≤45 years, female), at least 1 year of prior SSRI treatment, history of one to three MDEs, and prior treatment with sertraline, other SSRIs, or non-SSRI therapies were all factors that appeared to correlate with vortioxetine-mediated improvements in sexual functioning. Although the difference in treatment effect on CSFQ total score was statistically significant for each of the participant subgroups listed above, the sample sizes varied between groups of the same category (e.g., number of previous MDEs), making comparisons challenging. Further, the small number of men stratified by age limited our ability to determine TESD outcomes following antidepressant switch in this population, although it is known that men experience sexual dysfunction as age increases beyond mid-life.Reference Feldman, Goldstein, Hatzichristou, Krane and McKinlay32 Since this study was not powered to detect differences between the subgroups, further investigation will be required to determine whether these and any other participant-specific factors significantly affect vortioxetine-mediated improvements in sexual functioning.
Participants who directly switched from citalopram, sertraline, or paroxetine to escitalopram or vortioxetine maintained antidepressant efficacy as demonstrated by mean MADRS scores and remission rates. In participants switching from citalopram, numerically greater improvements in MADRS total scores were observed with vortioxetine compared with escitalopram. In the subpopulation of participants switching from paroxetine or sertraline, only modest differences were observed between treatment groups. However, interpretation of changes in MADRS total score among participants who had previously received paroxetine is limited by the small sample size, and the greater improvements observed with escitalopram vs. vortioxetine following switch from sertraline may reflect the greater baseline depression severity in the escitalopram group.
The findings from the present study demonstrated that in adults with well-treated MDD and SSRI-associated sexual dysfunction, an abrupt switch to vortioxetine once-daily therapy was a safe and viable approach for improving sexual functioning while maintaining antidepressant efficacy.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 Consistent with these findings, the results from the present analyses suggest that a direct switch from SSRI therapy to vortioxetine remains beneficial in terms of efficacy, and is not significantly impacted by prior SSRI treatment. With regard to safety and tolerability, although the direct switch to vortioxetine was tolerated in most individuals, in some, it was cause for study discontinuation. Nausea was temporary, regardless of treatment, and generally resolved after 14 days. However, higher rates of nausea were observed with vortioxetine than with escitalopram, and this effect persisted throughout the treatment period. The reported nausea was not surprising, as this adverse effect was also observed at similar frequencies during clinical development of vortioxetine. Nausea may be related to vortioxetine’s 5-HT1A receptor agonist activity, although it is worth noting that this same activity of vortioxetine may also contribute to the greater improvements in TESD with vortioxetine compared with escitalopram. Similar improvements in sexual function have also been noted with other multimodal antidepressants that act on the 5-HT transporter and presynaptic and postsynaptic 5-HT1A receptors, such as vilazodone.Reference Bang-Andersen, Ruhland and Jorgensen19, Reference Clayton, Gommoll, Chen, Nunez and Mathews33–Reference Rickels, Athanasiou, Robinson, Gibertini, Whalen and Reed35
While the incidence of TEAEs was generally comparable between treatment groups, nausea, dizziness and pruritus (for those who previously took citalopram) were more prevalent in the vortioxetine group. In clinical practice, however, the risk and severity of nausea, dizziness, and generalized pruritus with vortioxetine may be considered reasonable—especially when switching patients to agents that may ameliorate TESDs when previous antidepressants have failed to do so. Healthcare providers may also opt to consider additional strategies to mitigate the risk for nausea-related treatment discontinuations. Such strategies include initiating vortioxetine treatment at a lower dose such as 5 mg (and possibly cross-tapering while at this dose to avoid abrupt discontinuation of the previous SSRI) and/or uptitrating from the starting dose over a longer time period, instead of after only 1 week. In the present study, the increase to 20 mg vortioxetine was performed over this brief time period in order to ensure evaluation of antidepressant efficacy and sexual dysfunction at the highest approved dose. While there is a dose relationship with TESD at higher doses of vortioxetine, most subjects (75.9%) remained at the 20 mg dose during the study.Reference Jacobsen, Mahableshwarkar, Chen, Chrones and Clayton15 Thus, many options exist to address treatment-associated symptoms of nausea as well as those indicative of sexual dysfunction.
The interpretation of these findings is limited by the nature of the study design; only one active comparator was included in the primary study, and enrollment was not stratified by prior SSRI therapies, limiting our ability to detect statistically significant differences. The study was not powered for subgroup analyses of the primary, or efficacy, endpoints. Therefore, the relative clinical impact on depression symptoms of abruptly switching to vortioxetine vs. switching to another non-SSRI remains to be explored.
Conclusion
These analyses support the findings that switching SSRI antidepressant therapy to vortioxetine in well-treated adult MDD patients experiencing TESD can improve sexual dysfunction regardless of prior SSRI (citalopram, sertraline, and paroxetine), while maintaining antidepressant efficacy and tolerability. Overall, switching antidepressant therapy to vortioxetine appears to be a safe and effective alternative for patients experiencing sexual dysfunction during antidepressant therapy with an SSRI.
Acknowledgments
Medical writing assistance was provided by Gina M. DeStefano, PhD, Martina Schwarzkopf, PhD, and Andrea McReynolds, PhD, of inVentiv Medical Communications, LLC, a Syneos Health™ group company. This study was supported by the Takeda Pharmaceutical Company, Ltd., and H. Lundbeck A/S (NCT01364649). All the author(s) are entirely responsible for the scientific content of the paper.
Disclosures
John Affinito is an employee of Takeda Development Center Americas. Paula L. Jacobsen, Wei Zhong, and George G. Nomikos were employees at Takeda Development Center Americas at the time of study.
Andrew J. Cutler reports research support from or serving as a consultant to: AbbVie, Acadia Pharmaceuticals, Alkermes, Allergan, AstraZeneca, Avanir Pharmaceuticals, Axsome Therapeutics, Eli Lilly, Janssen, Intra-Cellular Therapies, Gedeon Richter, Janssen, Lundbeck, Novartis, Otsuka America Pharmaceutical, Alfasigma (Pamlab), Pfizer, Shire, Sunovion Pharmaceuticals, Takeda Pharmaceuticals, Teva Pharmaceutical Industries, and Vanda Pharmaceuticals; serving as a speaker for: Acadia Pharmaceuticals, Alkermes, Allergan, AstraZeneca, Lundbeck, Novartis, Otsuka America Pharmaceutical, Pamlab, Shire, Sunovion Pharmaceuticals, Takeda Pharmaceuticals, and Vanda Pharmaceuticals; serving on the board of the Neuroscience Education Institute.
Anita H. Clayton reports research support from or serving as a consultant to: Alkermes, AMAG Pharmaceuticals, Inc., Axsome Therapeutics, Endoceutics, Inc., Ivix, Janssen, Palatin Technologies, Sage Therapeutics, S1 Biopharma, Sprout Pharmaceuticals, Takeda Pharmaceuticals, and Valeant Pharmaceuticals; Royalties/Copyright from Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, Guilford Press; and shares/restricted stock units from Euthymics Bioscience, S1 Biopharma.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852919000750.