Introduction
Cognitive alterations and deficits that are observed in substance use disordersReference Gould 1 contribute directly and indirectly to the overall tremendous public health burden that these disorders place on society. Broadly, drug and alcohol use in human populations exists on a continuumReference Saha, Chou and Grant 2 , Reference Patrono, Gasbarri, Tomaz and Nishijo 3 ranging from nonpathological to levels of substance use diagnosed as a mental health disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM). 4 Here, we discuss cognitive changes that are on the spectrum where drug use already represents a disorder. This type of drug use can be defined as a “pathological pattern of behaviors related to use of the substance,” characterized by a compulsive and chronically relapsing pattern of drug use, impaired control over substance use, continuation of use despite negative consequences, craving, tolerance, and withdrawal. 4 , Reference Koob and Volkow 5 The typical cognitive domains contributing to this understanding of addiction are attention, response inhibition, decision-making and working memory.
Recently a new systemic conceptual framework for neuroscience, the Research Domain Criteria (RDoC) National Institutes of Health initiative, was launched.Reference Insel, Cuthbert and Garvey 6 RDoC is a framework for analyzing mental processes, wherein disorders are considered in terms of disruptions along the continuum of normal to pathology across the full range and along the elemental psychological processes and behavioral functions. This approach is increasingly being used in research, where an appreciation and understanding of its utility is building. RDoC is applied transdiagnostically along the continuum of normal–pathology for the domain or construct in question, allowing one to step away from categorical diagnoses.Reference O’Donnell and Ehlers 7
The RDoC approach has recently been applied to substance use disorders.Reference Kwako, Momenan, Litten, Koob and Goldman 8 Three addiction-relevant domains were highlighted—executive function, incentive salience, and negative emotionality.Reference Koob 9 These functional domains roughly correspond to classical stages in the addiction cycle, and they can also be viewed as concurrent contributors to addiction and relapse vulnerability (see Figure 1). These three domains, intended to cover the core elements of the addiction cycle as a disorder, can be measured across a variety of substance use disorders. In this review we will focus on the executive (cognitive) domain, providing an overview of impairments that may serve as intermediate phenotypes for (behavioral, pharmacologic, or neurostimulatory) intervention.
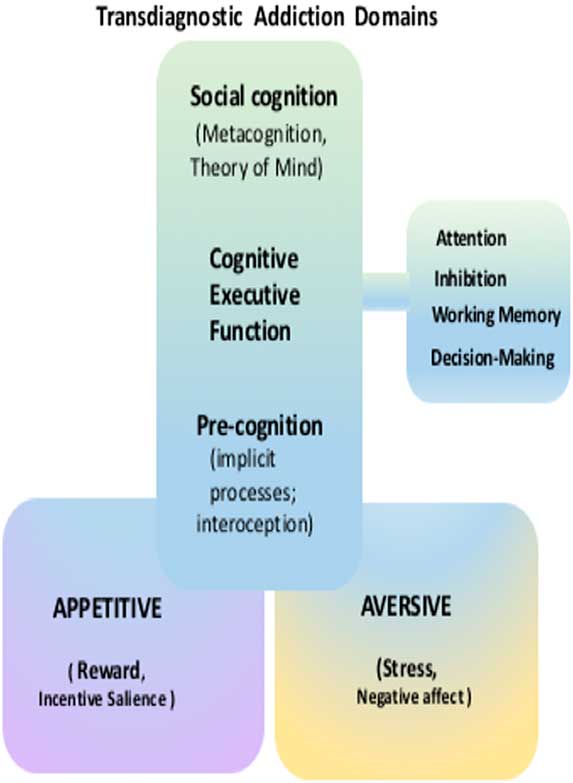
Figure 1 A visual representation of several transdiagnostic research domains of addiction. Substantial prior research has been conducted on fundamental alterations in the appetitive and aversive motivations processes. The focus of the current paper is a continuum of cognition, ranging from precognitive (implicit) processes to classical executive function (attention, inhibition, working memory, and decision-making) and social cognition (metacognition theory of mind).
This overview proposes to extend the three established cognitive domains in substance use disorders to include (1) precognition, featuring processes that occur outside or before conscious cognition per se; and (2) social cognition, including metacognition/insight theory of mind (ToM). These expanded domains may be an integral part of the human addiction phenotype and could potentially hold the key to aspects of the addiction phenotype that make treatment and functional impairments in substance use disorders so challenging (Figure 1).
Several models of addiction address cognitive impairments that either predispose a person to addiction or result from drug exposure. For example, Goldstein and VolkowReference Goldstein and Volkow 10 proposed a model in which disrupted cortical top-down processes are a result of prefrontal cortex (PFC) dysfunction that leads to impaired response inhibition and salience attribution.Reference Goldstein and Volkow 10 , Reference Goldstein, Volkow, Wang, Fowler and Rajaram 11 In other words, there is a decreased ability to modify behavior related to drug and drug cues (response inhibition impairment) coupled with an abnormal salience (salience attribution change) of drug and drug-related cues.Reference Goldstein and Volkow 10 Other models emphasizing cognition include Monterosso and Ainslie’s description of impulsive choice in humans as a hyperbolic (rather than exponential) function.Reference Monterosso and Ainslie 12 They argue that impulsive behaviors (e.g., the preference for smaller, more immediate rewards vs. larger, more delayed rewards) often result from a breakdown of cognitive self-control mechanisms. Bickel and MarschReference Bickel and Marsch 13 expanded on this model and applied it to addiction, showing that individuals with addiction discount delayed rewards to a much greater extent than healthy controls.Reference Madden, Petry, Badger and Bickel 14 Sofuoglu and colleaguesReference Sofuoglu, DeVito, Waters and Carroll 15 proposed a dual-process model, wherein a balance between “top-down” and “bottom-up” processes compete for control of behavior. They argue that neurobiological bottom-up processes are implicit and more automatic, and when heightened, increase the risk of drug use and relapse. Executive top-down processes, impaired in individuals with addiction, are more deliberative and are responsible for modulating the downstream (more automatic) processes.
Attention
Addiction is characterized by a strong attentional preference for drugs and drug-related cues.Reference Field, Mogg and Bradley 16 – Reference Anderson 18 Attentional biases are often implicitReference Wetherill, Childress and Jagannathan 19 and occur automatically.Reference Field and Cox 20 Some researchers suggest that drug-related cues gain positive incentive properties through a classical conditioning process,Reference Stewart, de Wit and Eikelboom 21 , Reference Robinson and Berridge 22 while other researchers posit that negative affect can increase the salience (“attention-grabbing” quality) of drug-related cues,Reference Baker, Piper, McCarthy, Majeskie and Fiore 23 both of which facilitate drug-seeking behaviors. Whether established through positive or negative motivation, attentional bias can then drive drug seeking,Reference Leeman, Robinson, Waters and Sofuoglu 24 reflected in a shift of salience for drug-related cues and behavioral resources being directed toward the goal of drug consumption.
There are several tasks used to measure attentional bias. The Stroop interference task asks individuals to name the font color of several words; interference occurs when the content of the word is different from the font or is emotionally charged (drug words),Reference Cox, Fadardi and Pothos 25 resulting in a slower reaction time. Generally, users of nicotine,Reference Froeliger, Modlin, Wang, Kozink and McClernon 26 – Reference Rzetelny, Gilbert, Hammersley, Radtke, Rabinovich and Small 28 cocaine,Reference Pike, Stoops, Fillmore and Rush 29 , Reference Waters, Marhe and Franken 30 heroin,Reference Waters, Marhe and Franken 30 – Reference Franken, Kroon, Wiers and Jansen 33 cannabis,Reference Field 34 , Reference Cousijn, Watson, Koenders, Vingerhoets, Goudriaan and Wiers 35 and alcoholReference Snelleman, Schoenmakers and van de Mheen 36 have slower reaction times when encountering words associated with their substance use disorders. Visual attention tasks can also reveal accelerated reaction times to drug-related stimuli.Reference Mogg, Bradley, Field and De Houwer 37 – Reference Jones, Bruce, Livingstone and Reed 40 Illustrating that attention may drive approach, other tasks have measured the attention-driven approach to drug-related cues using a “joystick” procedure. In these tasks, participants either push (avoidance) or pull (approach) a joystick in response to drug-related cues, whether explicit or implicit.Reference Wiers, Eberl, Rinck, Becker and Lindenmeyer 41
Researchers have begun using these tasks to alter attentional biases (e.g., attentional bias modification training) in an attempt to decrease drug use. Results have shown that attentional bias modification can reduce bias to alcohol cuesReference Wiers, Eberl, Rinck, Becker and Lindenmeyer 41 – Reference Schoenmakers, de Bruin, Lux, Goertz, Van Kerkhof and Wiers 44 but not cocaine cues.Reference Mayer, Wilcox and Dodd 45 Thus far, results are modest, with the field still actively researching the most effective way to alter attentional bias to substance-related cues—and to translate these attempted changes into clinical outcomes.Reference Wiers, Gladwin, Hofmann, Salemink and Ridderinkhof 46 The underwhelming clinical results underscore the entrenched nature of attentional bias to drug-related cues and their potential importance as a treatment target.
Response Inhibition
Loss of control over drug use is a key feature of addiction. 4 Inhibitory control refers generally to the ability to suppress or counter responses—whether these responses are behaviors, thoughts, or motivational states. In addiction, impairments in inhibitory control may account for the fundamental difficulty in resisting the motivational “pull” of drugs, thus increasing the vulnerability to relapse.Reference Smith, Mattick, Jamadar and Iredale 47 , Reference Luijten, Field and Franken 48 Poor inhibitory control may also account for several behavioral patterns that are common in substance use disorders, including increased impulsivity,Reference Bornovalova, Daughters, Hernandez, Richards and Lejuez 49 – Reference Verdejo-Garcia, Rivas-Perez, Vilar-Lopez and Perez-Garcia 51 increased sensation seeking,Reference Patkar, Murray, Mannelli, Gottheil, Weinstein and Vergare 52 increased risk-taking for rewards,Reference Lejuez, Aklin and Jones 53 , Reference Lejuez, Aklin, Zvolensky and Pedulla 54 and poor decision-making (e.g., choosing small immediate rewards over delayed, larger rewards),Reference Bechara, Dolan, Denburg, Hindes, Anderson and Nathan 55 discussed in more detail later. Neuropsychological and neuroimaging studies have localized inhibitory control circuitry in the PFCReference Garavan, Hester, Murphy, Fassbender and Kelly 56 ; these circuits normally function in a top-down way to govern downstream motivational systems for drugs and natural rewards.Reference Smith, Mattick, Jamadar and Iredale 47 , Reference Childress, Mozley, McElgin, Fitzgerald, Reivich and O’Brien 57 , Reference Kaufman, Ross, Stein and Garavan 58 This ability to inhibit impulses toward reward, to delay gratification, shows significant variability across individuals, and these differences can be detected very early in development,Reference Mischel, Shoda and Rodriguez 59 well before any drug exposure. Importantly, however, chronic exposure to some drug classes (especially stimulants) can actually erode the “braking ability” of the brain.Reference Goldstein and Volkow 60 – Reference Garavan, Kaufman and Hester 62
The most common tasks for probing inhibitory control feature instructed attempts to inhibit a simple prepotent motor response. The “go/no-go” task presents a stream of “go” stimuli (often simple letters or shapes) that require a rapid button press, while infrequent “no-go” stimuli require “withholding” the button press.Reference Horn, Dolan, Elliott, Deakin and Woodruff 63 Accidentally pressing the button to a no-go target indicates a failure to inhibit. “Stop-signal” tasks require the inhibition of a motor response that is already underway,Reference Robbins 64 , Reference Dalley and Robbins 65 with reaction time (to stop) as the primary measure. Though these motor tasks are simpler than real-world situations requiring inhibition (e.g., resisting drug use), poor performance in these tasks is generally well correlated with higher-order failures of inhibition, such as drug relapse.Reference Moeller and Paulus 66 In an attempt to capture real-world inhibition struggles, some tasks have used valenced stimuli as signals, requiring inhibition of approach to “positive” stimuli,Reference Casey, Somerville and Gotlib 67 , Reference Goldman, Ehrman and Suh 68 and responding in a valenced go/no-go task was actually better correlated with clinical symptom severity (impulsivity in attention-deficit hyperactivity disorder)Reference Goldman, Ehrman and Suh 68 than responding in standard non-valenced go/no-go tasks.
Laboratory models that require participants to attempt inhibition of craving to drug video cuesReference Lam, Wang and Li 69 provide a close parallel to the real-world challenges faced by patients in recovery. These paradigms reveal that patients with a better outcome prognosis demonstrate good communication (functional connection) between cortical inhibitory regions (i.e., the dorsal anterior cingulate) and downstream motivational circuitry (e.g., the amygdala), whereas patients with a poor outcome prognosis lack this critical connection. In general, the neuroimaging literature has identified poorer recruitment (hypoactivity) of top-down inhibitory regions in drug users versus controls during simple laboratory tasks of inhibition; this is especially marked in stimulant users.Reference Smith, Mattick, Jamadar and Iredale 47 , Reference Luijten, Field and Franken 48 , Reference Garavan, Hester, Murphy, Fassbender and Kelly 56 , Reference Kaufman, Ross, Stein and Garavan 58 , Reference Spechler, Chaarani, Hudson, Potter, Foxe and Garavan 70 – Reference Luo, Zhang and Hu 72 Intriguingly, cocaine patients who achieve extended abstinence actually demonstrate a heightened ability to recruit cognitive control regions.Reference Connolly, Foxe, Nierenberg, Shpaner and Garavan 73 This could suggest either that recovery of inhibitory ability improves with (cocaine) abstinence and/or that individuals with strong inhibitory ability are more likely to achieve extended abstinence.Reference Bell, Foxe, Ross and Garavan 74 Longitudinal studies will be needed to address these possibilities.
Studies attempting to improve inhibitory function either with medications that target frontal circuitryReference Robbins 64 , Reference Dalley and Robbins 65 or by direct neural stimulation (Transcranial Magnetic Stimulation or transcranial direct current stimulation) of inhibitory circuitryReference Jacobson, Javitt and Lavidor 75 , Reference Dunlop, Hanlon and Downar 76 are still in the early stages. However, they offer continued encouragement that inhibition is a clinically meaningful intermediate phenotype for targeted interventions.
Working Memory
Baddeley defined working memory as a system for the temporary maintenance and manipulation of information, necessary for the performance of such complex cognitive activities as comprehension, learning, and reasoning.Reference Baddeley 77 It is thought to include three subsystems: a phonological loop, concerned with verbal and acoustic information; a visuospatial sketchpad, concerned with visual information; and the central executive, a capacity-limited control system that allocates finite resources and actively manipulates them.Reference Baddeley and Hitch 78 , Reference Baddeley 79
Tasks that measure working memory (n-back, visuospatial, digit and word recall, verbal memory, etc.), have revealed cognitive deficits in individuals with a substance use disorder.Reference Goldstein and Volkow 10 , Reference Bechara and Martin 80 – Reference Goldstein, Leskovjan and Hoff 85 Working memory impairments could be associated with chronic toxic effects of drug use,Reference Yan, Li, Xiao, Zhu, Bechara and Sui 84 and lower executive cognitive ability has been found to increase susceptibility to problematic drug use.Reference Hester, Lubman and Yücel 86 As such, working memory represents a therapeutic target in substance use disorders that could be potentially linked with functional outcomes. Retraining working memory may help to bolster the central executive subsystem of working memory, which may help other cognitive functioning.Reference Baddeley 79 Thus, addiction researchers have begun to target working memory with the goal of improving cognitive control. For example, Bickel and colleagues used a program (e.g., verbal memory, recall of numbers and words) to train the working memory of individuals with stimulant use disorders.Reference Bickel, Yi, Landes, Hill and Baxter 82 Those authors were able to show improvements of delay-discounting, but not working memory. Houben and colleagues used working memory training on individuals with alcohol use disorders and found both reduced alcohol use and improved working memory.Reference Houben, Wiers and Jansen 87
Another rationale for targeting working memory relates to dopaminergic mechanisms, known to be central to addiction.Reference Volkow, Fowler, Wang, Baler and Telang 88 Working memory capacity is dependent on dopaminergic mechanisms,Reference Cohen, Braver and Brown 89 and it has been shown that working memory training affects dopamine systems.Reference McNab, Varrone and Farde 90 , Reference Backman, Nyberg and Soveri 91 When behavioral interventions are not fully effective, having pharmacological approaches could act as a facilitation tool. For example, there is accumulated evidence that working memory impairments might be compensated with psychoactive drugs,Reference Sofuoglu, DeVito, Waters and Carroll 92 – Reference Joyce and Millan 94 optimizing dopaminergic function in individuals with addiction. In turn, this may aid them in achieving long-sought functional restoration and support the goals of drug use reduction and abstinence.
Decision-Making Systems
Seemingly poor decision-making is a prominent feature of addiction, reflected in the continued use of drugs and alcohol in the face of negative consequences. 4 This type of behavior may seem counterintuitive, but there are several theories that address why/how these “poor” choices continue to be made. Verdejo-García and Bechara’s “somatic markers” theoryReference Verdejo-García and Bechara 95 states that individuals with addiction have reduced awareness of learned emotional warning signals from the body (interoception) that translates into risky decision-making and “myopia” for the future, similar to individuals with ventromedial PFC lesions. Similarly, Bickel and Marsch focus on cognitive impairment that leads to a discounting of larger, delayed rewards for a preferred smaller, more immediate reward.Reference Bickel and Marsch 13 These poor decisions are thought to arise from an imbalance of top-down and bottom-up processing. Top-down processing involves deliberative decisions, which are flexible and sensitive to devaluation, but are slow and cognitively intensive.Reference van der Meer, Kurth-Nelson and Redish 96 On the other hand, more automatic actions, such as habit-based and classically conditioned behaviors are fast but inflexible and insensitive to devaluation.Reference van der Meer, Kurth-Nelson and Redish 96 Initially, decisions to use drugs and alcohol are more deliberate, but with continued use, these actions will transition into more automatic behaviors, eventually becoming compulsive.Reference Teper and Inzlicht 97 Contributing to this transition to more automatic decision-making is (learned) incentive salience, the tendency of drug-related cues to take on motivating properties.Reference Robinson and Berridge 22
Several tasks are used to measure aspects of decision-making systems. For example, delay-discounting tasks allow for the assessment of how well someone is able to delay immediate gratification for a higher-value reward later.Reference Bickel and Marsch 13 Individuals with addiction tend to discount larger, delayed rewards more than healthy controls, and these higher rates of discounting larger, future rewards have been shown to be associated with disadvantageous behaviors,Reference Bickel and Marsch 13 including drug use. Other tasks, such as the Iowa gambling task measure real-time decision-making, and people with addiction generally perform worse than controls; a subgroup of addicted individuals may lack the implicit interoceptive guidance toward a more advantageous strategy.Reference Bechara, Damasio, Tranel and Damasio 98
Researchers have adapted several methods in an attempt to restore the balance of top-down and bottom-up processing. For example, working memory training (as discussed earlier) seems to bolster the central executive subsystem, reducing discounting,Reference Bickel, Yi, Landes, Hill and Baxter 82 improving working memory,Reference Houben, Wiers and Jansen 87 and decreasing substance use.Reference Houben, Wiers and Jansen 87 Recently, meditation has shown promise as a way to potentially improve executive controlReference Teper and Inzlicht 99 and to improve awareness of internal states (and the ability to label them), thus countering alexithymiaReference Baer, Smith, Hopkins, Krietemeyer and Toney 100 and potentially improving interoception. Contingency management approaches may boost deliberative decision-making and help reduce automated drug-choice behaviors by making concrete non-drug rewards immediately availableReference Regier and Redish 101 and contingent on a reduction in drug use. Contingency management approaches have good impact while the procedures are in place,Reference Stitzer and Bigelow 102 – Reference Petry, Martin and Finocche 104 with the transition to the broader real-world setting as the clinical challenge. Preclinical research has demonstrated that automatic behaviors are malleable (e.g., inactivating parts of the brain that underlie habit-based behaviors [e.g., dorsolateral striatum] reduces automatic actions and increases cognitive regulation by other brain areas [e.g., hippocampus]).Reference Packard and McGaugh 105 In humans, devaluation of drug-related stimuli can reduce drug use behaviors,Reference Houben, Havermans, Nederkoorn and Jansen 106 also pointing to the potential modification of decision-making with behavioral strategies. However, given the reflexive nature of automated decision processes—not just in addiction pathology, but in everyday decision-making—the clinical impact of behavioral strategies is often modest and has encouraged the testing of additional approaches. Direct neurostimulation of cortical areas via transcranial magnetic stimulation offers a promising approach, as it has been shown to reduce craving Reference Hanlon, Dowdle and Austelle 107 , Reference Wing, Barr and Wass 108 and delay discounting.Reference Cho, Koshimori and Aminian 109 Pharmacologic approaches to improve decision-making with “cognitive enhancers” also offer preliminary evidence that is promising. For example, modafinil (dopamine drug with potential abuse liability) improved delay discounting,Reference Schmaal, Goudriaan and Joos 110 and atomoxetine (non-dopamine drug without abuse liability) improved impaired executive function.Reference Brown, Holdnack and Saylor 111 Even though a clinical trial of atomoxetine in cocaine addiction was disappointing,Reference Walsh, Middleton and Wong 112 there is an ongoing need for medications that can either reduce the implicit, automated processes in decision-making, bolster the deliberative processes, or both.
Precognition
Processes that are rapid and implicit and that even occur outside conscious awareness are important precursors and contributors to each of the classic executive cognitive domains reviewed here (attention, inhibition, working memory, and decision-making). In Figure 1, processes in the precognitive realm are shaded in blue, and can originate from appetitive or aversive motivational states. Though some of these may eventually be reflected in an explicit (shaded in green) cognition or decision, for example, “I will plan to buy drugs when I get paid tomorrow,” others may shape drug-related feelings and behavior while remaining completely outside awareness.
In the domain of attention, the response to drug cues is fast, involuntary, and implicit – the product of powerful prior associative learning. The individual struggling with a substance use disorder does not need to consciously, deliberately focus attention on a drug-related cue for it to have a motivating effect.Reference Wetherill, Childress and Jagannathan 19 , Reference Fadda, Scherma, Fresu, Collu and Fratta 116 Indeed, the riveted attention to a drug-related cue may occur even when successful task performance instead depends on a flexible shift of attention away from drug images.Reference Field, Mogg and Bradley 16 , Reference Field and Cox 20 Precognitive processes also play a role in inhibition tasks. As previously detailed, these tasks typically instruct deliberate, explicit, conscious attempts to inhibit a “prepotent” (whether motor- or drug-related) response. However, the prepotency of the responses to be inhibited depends on their “near-automatic” nature. For example, motor prepotency results from a rapid series of button presses to a “go” signal, and a prepotent approach to drug stimuli is the near-automatic result of much prior learning. In the domain of working memory, an individual’s ability to maintain and update information, to allocate cognitive resources, generally happens implicitly, from moment to moment, without a conscious focus. Experimental tasks that probe working memory may instruct the participant to intentionally recall an item earlier in a string (e.g., the n-back test), but in real life, this kind of memory occurs in an ongoing precognitive way, without explicit awareness and without prompting. The realm of decision-making especially highlights the “competition” between fast, implicit, precognitive responses (e.g., the approach response to immediate reward and discounting of delayed future rewards) versus slower, deliberative responses (e.g., taking the future into account, including any future negative consequences of the approach to the drug reward). The human challenge of balancing fast, implicit, precognitive decision processes against slow, deliberative processes has been recognized across history and is the foundation for several “dual-process” modelsReference McClure and Bickel 113 of decision-making.
Given the broad contribution of precognitive processes, what are the implications for addiction treatment? As conventional cognitive behavioral treatments are directed to faulty explicit cognitions, these interventions may not affect implicit processes. That the high rates of relapse that are common for substance use disorders have remained relatively unchanged across the decades may reflect (at least in part) a difficulty in addressing the precognitive domain. As noted earlier, from the few available studies, behavioral attempts to change the attentional bias to drug cues have met with only modest success,Reference Wiers, Eberl, Rinck, Becker and Lindenmeyer 41 and studies using working memory training are still in the early stages.Reference Bickel, Yi, Landes, Hill and Baxter 82 , Reference Houben, Wiers and Jansen 87 Encouragingly, pharmacologic interventions might be well suited to the precognitive domain. As an example, a recent study demonstrated that the noradrenaline uptake inhibitor atomoxetine was able to reduce attentional bias to cocaine cuesReference Passamonti, Luijten and Ziauddeen 114 (though a clinical trial did not demonstrate benefitReference Walsh, Middleton and Wong 112 ), and the opioid antagonist naltrexone was shown to improve the recruitment of modulatory circuitry (lateral orbitofrontal cortex) in a “now–later” decision-making task.Reference Boettiger, Kelley, Mitchell, D’Esposito and Fields 115 The GABAB agonist baclofen, known to reduce dopamine release,Reference Fadda, Scherma, Fresu, Collu and Fratta 116 was shown to blunt the mesolimbic activation triggered by 33msec cocaine cues presented outside conscious awareness.Reference Young, Franklin and Roberts 117 The ability of brief “unseen” drug cues to trigger motivational circuitryReference Childress, Ehrman and Wang 118 , Reference Wetherill, Young and Jagannathan 119 offers a paradigm for screening the ability of candidate medications to impact precognition, complementing conventional self-reports of conscious motivational (“craving”) states.
A construct with a special relationship to the precognition domain is interoception, the organism’s sense of its own internal state(s).Reference Schmidt, Eulenbruch, Langer and Banger 120 – Reference Craig 122 Broadly, interoception is based on bodily sensations reflecting a change in internal state (e.g., hunger, thirst, temperature) or autonomic visceral responses (e.g., heart palpitations, sweating, gut motility) arising in response to powerful external stimuli (e.g., pain, threat, sexual opportunity, even the anticipated reward from drugs of abuse). Importantly, these varied bodily sensations can also become attached, through learning, to previously neutral cues, enabling the cues to guide the organism away from danger, or toward reward.Reference Craig 121 , Reference Craig 123 , Reference Paulus and Stewart 124
With these links both to danger and to reward, it is understandable that interoception has been featured in human addiction models. Bechara and colleaguesReference Bechara, Damasio, Tranel and Damasio 98 hypothesized that impaired interoception (aka “somatic markers”) for negative stimuli (e.g., the negative consequences of drug choice) could contribute to relapse. On the other hand, heightened interoception for the positive (appetitive) arousal triggered by drug reminder cues can also be a relapse vulnerability,Reference Childress, Mozley, McElgin, Fitzgerald, Reivich and O’Brien 57 , Reference Childress, Ehrman and Wang 118 , Reference Bechara, Dolan and Hindes 125 fueling the “incentive salience” of these cues. Whether for aversive or appetitive states, the anterior insula has been strongly implicated in interoceptive processing and emotional awareness.Reference Wetherill, Young and Jagannathan 119 Supporting the clinical significance of the insula in interoception, an attenuated response in the insula during decision-making predicted relapse in methamphetamine users.Reference Paulus, Tapert and Schuckit 126 , Reference Gowin, Harle, Stewart, Wittmann, Tapert and Paulus 127 Intriguingly, cigarette smokers who sustained injury to the insula—presumably impacting both appetitive and aversive interoceptions—lost the motivation to smoke (they “simply forgot to crave a cigarette”).Reference Lawrence, Su and Barker 128 , Reference Veit, Singh, Sitaram, Caria, Rauss and Birbaumer 129 As in these examples, interoceptions often have their origins in the precognitive domain and can influence addiction-relevant decision-making, even when—perhaps especially when—the individual has limited self-awareness. Indeed, some therapeutic approaches in substance use (and other disorders) are geared to improving the individuals’ conscious, explicit awareness of their internal states as a step toward greater cognitive control. Novel treatments targeting the insula with real-time neuro-feedbackReference Lawrence, Su and Barker 130 – Reference Lee, Ruiz, Caria, Veit, Birbaumer and Sitaram 132 or direct brain stimulationReference Pushparaj, Hamani and Yu 133 underscore the promise of interoceptive processes as a meaningful therapeutic target in substance use disorders.
Social Cognition
Metacognition
We humans have the ability to look inside ourselves, which allows us to understand the relationship between ourselves and others, to monitor our own thought processes, and to control thoughts, all of these activities are related to metacognition. However, impairments of metacognition can have negative consequences on decision-making, such as being overconfident about a poor decision or lacking confidence in a better decision.Reference Batha and Carroll 134 , Reference Hauser and Allen 135
The scope of metacognitive impairment in substance use disorders has not been well researched, despite it being a striking and critical feature of the addiction phenotype. For example, many researchers report a dissociation between self-report and behavior, low treatment compliance, frequent relapse, impaired psychosocial functioning, and a lack of perception that treatment is actually needed. In 2015, more than 21 million individuals (12 or older) were classified as needing treatment for a substance use disorder. Just more than 10% actually received treatment for a disorder; however, among the rest (∼19 million), only about 5% perceived the need for treatment.Reference Lipari, Park-Lee and Van Horn 136 Goldstein and colleagues have reported that this impairment is reflective of an existing dysfunction in the neural circuitry.Reference Goldstein, Craig and Bechara 137 Neurologically, it is thought that mechanisms of metacognition reside in frontal structures, such as the rostral anterior cingulate cortex,Reference Moeller, Fleming and Gan 138 , Reference Moeller, Konova and Parvaz 139 and that dysfunction of ventrolateral PFC may be an important contributor to the insight impairment.Reference van der Meer, de Vos and Stiekema 140
Metacognitive deficits can be thought of as impairments of insight, shown to be a common feature in addiction.Reference Balconi, Finocchiaro and Campanella 141 , Reference Brevers, Cleeremans and Bechara 142 In the substance use disorder literature, lack of insight has sometimes been conflated with “denial.” However, the two are distinct. Denial implies a refusal or contradiction of something of which the person is aware, while lack of insight involves a lack of awareness of something present in the individual. Some researchers in mental health disorders separate clinical insight, which is a construct composed of awareness of illness, recognizing the need for treatment, and relabeling symptoms, and impaired general insight, which is connected with poorer treatment outcome, an inability to perceive the severity of illness, poor psychosocial functioning, higher relapse, and low self-esteem.Reference Amador and Davis 143 If individuals with substance use disorders are not able to assess the level of severity of their impairments, or in some cases are not even aware that they have a disorder, this may help explain the lack of perceived need for treatment. It is worth noting that even after recognizing the need for help and seeking treatment, patients may still struggle and relapse. Thus self-awareness is important but maybe not sufficient for recovery.
It has also been noted that addiction involves deficits of self-awareness and behavioral control similar to what is seen in other neuropsychiatric disorders (e.g., mood, psychotic, and neurological disorders).Reference Goldstein, Craig and Bechara 137 , Reference Orfei, Robinson, Bria, Caltagirone and Spalletta 144 , Reference Orfei, Piras and Banaj 145 Research has suggested that this insight deficit is reflected in one of the key attributes of substance use disorder defined in the DSM classification: drugs are used despite negative consequences. Self-awareness deficits and metacognitive impairments persist even in remitted drug users, revealed, for example, by remitted users’ poor association between self-reported confidence in performance and actual performance on a visuo-perceptual accuracy task.Reference Moeller, Bederson, Alia-Klein and Goldstein 146
We are aware of at least two methods to address the insight deficit and bolster metacognitive abilities. One is metacognitive therapy (MCT), which was first developed to address impairments of cognition that occur in several stress-related disorders such as depression, anxiety, PTSD, and obsessive-compulsive disorder.Reference Spada, Caselli, Nikcevic and Wells 147 – Reference Fisher and Wells 152 Metacognitive therapy has been described a hybrid of cognitive-behavioral therapy and psychoeducationReference Moritz, Woodward and Balzan 153 , Reference Spada, Caselli, Nikčević and Wells 154 and has been shown to be efficacious in reducing schizophrenia-related anxiety and depression symptoms.Reference Eichner and Berna 155 , Reference Normann, van Emmerik and Morina 156 Another method is metacognitive strategy instruction, which was found to be helpful for those with below-average decision-making performance but not for those with average or above-average decision-making performance.Reference Lipari, Park-Lee and Van Horn 136 Recent metacognitive models have been directed toward addiction,Reference Wells and Simons 148 , Reference Spada and Wells 157 but formal clinical trials in substance use disorders using MCT or metacognitive strategy instruction are not yet available.
Theory of mind
Within the social cognition domain of addiction, there exists a relatively understudied cognitive construct called theory of mind (ToM). ToM is described as the cognitive capacity to have an implicit assumption about the behavior and intentions of others, as driven by their desires, attitudes, and beliefs.Reference Premack and Woodruff 158 – Reference Mitchell and Phillips 160 The capacity for social insight in humans is dependent upon this process. ToM mainly consists of two subtypes: “cognitive” ToM for attribution of beliefs and intentions and “affective” ToM for attribution of emotions.Reference Bosia, Riccaboni and Poletti 161 Studies of ToM and its impairment in mental disorders traditionally were investigated in developmental psychology in childrenReference Flavell 162 , Reference Flavell 163 but then were applied to disorders such as autism, schizophrenia, and personality and neurological disorders, in which impairments in social cognition are very central to their phenotypical presentation.Reference Tay, Hulbert, Jackson and Chanen 159 , Reference Ciaramidaro, Bölte and Schlitt 164 – Reference Downey, Blezat and Nicholas 168 In psychotic spectrum disorder and schizophrenia, for example, social cognition impairment has been strongly linked with functional outcome.Reference Schmidt, Mueller and Roder 169 – Reference Barbato, Liu and Penn 171 In addition, evidence has accumulated that ToM mediates the pathway from neurocognition to functional outcome in young adults with recent onsets of mood, anxiety, and personality disorders,Reference Francesconi, Minichino and Carrión 172 and in people with bipolar disorders, ToM deficits could be viewed as a core deficit feature, which is independent from other symptoms and patient characteristics.Reference Pluta, Kulesza, Grzegorzewski and Kucharska 173 For substance use disorders, recent meta-analysis of social cognition in alcohol use disorder showed a significant deficit in emotion recognition and cognitive ToM.Reference Bora and Zorlu 174 ToM impairments were also found in individuals with cocaine use disorders Reference Sanvicente-Vieira, Kluwe-Schiavon, Corcoran and Grassi-Oliveira 175 but not recreational cocaineReference Kemmis, Hall, Kingston and Morgan 176 or cannabis users.Reference Preller, Hulka and Vonmoos 177 , Reference Roser, Lissek, Tegenthoff, Nicolas, Juckel and Brüne 178 More sensitive neurophysiological measures of brain activity (e.g., fMRI), may be able to further identify differences in the ToM neural network activation of individuals using substances recreationally and those with clinically diagnosable substance use disorders.
In regard to assessment tasks, the “Reading the Mind in the Eyes” task has been used to examine affective ToM.Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb 179 The “Theory of Mind Stories” task has been used to examine second-order understanding of false beliefs,Reference Shamay-Tsoory, Shur, Barcai-Goodman, Medlovich, Harari and Levkovitz 180 and there are other tasks in development (e.g., using a virtual reality paradigmReference Canty, Neumann and Shum 181 ). Interventions that have shown to bring positive change in affective ToM are possible, including psychodynamic art therapy, which was previously shown to reduce symptoms in patients with schizophrenia.Reference Montag, Haase and Seidel 182
Social cognition impairments are present transdiagnostically across neuropsychiatric disorders, including substance use disorders. However, more research in social cognition deficits for substance use disorders is needed to potentially add treatment options that may translate into long-sought functional gains.
Conclusion
This review presents an overview of cognitive impairments in drug and alcohol use disorders. Cognitive impairments are addressed as a continuum, with one end representing the more precognitive processes and the other end extending to higher levels, such as social cognition; in between are the familiar cognitive executive domains (Figure 1). In this review of the cognitive executive domain, we found that most of the research has been focused on characterizing patients versus controls and documenting differences in each of these domains (e.g., attention, response inhibition, working memory, decision-making systems). For each of these domains, there is also an emerging body of evidence for its status as an intermediate phenotype and potential target for intervention. However, the translation from intermediate phenotype to clinical outcome is still in the early stages. Novel treatments (e.g., neurostimulation, pharmacologic “rebalancing”) offer promise for the next phase of translational research, inclusive of cognitive deficits in substance use disorders. We described a “cognitive continuum,” for which the extremes (precognition, social cognition) have been less studied. We suggest the unique features of these domains (e.g., impaired interoception, metacognitive deficits, impaired insight into illness) offer viable therapeutic targets that may both require and stimulate entirely new interventions.
It is important to recognize that, in cross-sectional research, it is difficult to determine whether drug use was predated, predisposed, exacerbated, or caused entirely by cognitive impairments. New longitudinal studies in developmental cohortsReference Lisdahl, Sher and Conway 183 before drug exposure will help to determine the relative contribution of individual variables (e.g., genetics, epigenetics, adversity) versus drug variables (e.g., type of drug, dose, exposure, frequency) to the observed impairments. This information is critical both for selecting therapeutic targets and for shaping therapeutic expectations (e.g., restoring function vs. remedial biological supports).
Finally, worth noting, the phenotypical features stemming from both the familiar and extended cognitive domains are not confined to addiction but are both dimensional and transdiagnostic and relevant for other neuropsychiatric disorders and conditions (a focus of RDoC). Thus, therapeutic discoveries in the addiction arena might be expected to have direct relevance for other major psychiatric disorders sharing the dimensions of these cognitive impairments.Reference Kwako, Bickel and Goldman 184 Research in these domains will also be helpful in empirically determining the unique contributions of intermediate phenotypes versus an overall psychopathology (e.g., factor “p”Reference Caspi, Houts and Belsky 185 ) in guiding treatments and predicting clinical outcomes.
Acknowledgments
The authors wish to acknowledge Anna Rose Childress, PhD, for input on precognition and Figure 1, and to thank Kimberly Young, PhD, and Stefanie Darnley, BS, for professional assistance in preparing the article for submission. Professional research effort for Paul S. Regier was supported by a National Institute on Drug Abuse (NIDA) T32 Translational Addiction Research Training program (Childress, Co-PI); professional effort for Dr. Childress, Dr. Young, and Ms. Darnley was supported in part by a NIDA U54 Cocaine Cooperative Medication Development Center (U54DA039002, Kampman PI) and by NIDA R01 DA039215 (Childress, PI).



