Take-Home Points
1. Vortioxetine is an antagonist at serotonin (5HT) 5HT3 receptors.
2. Vortioxetine also targets the serotonin transporter (SERT), 5HT1A, 5HT1B, 5HT1D, and 5HT7 receptors.
3. 5HT3 antagonism enhances not only the release of 5HT, but also of norepinephrine (NE) and acetylcholine (ACh), which may be linked to its antidepressant and procognitive properties.
Vortioxetine is a “multimodal” agent that simultaneously acts at 6 pharmacologic targets with 3 modes of action (Figure 1)Reference Mørk, Pehrson and Brennum1–Reference Sanchez, Asin and Artigas4:
1. Inhibition of the serotonin (5HT) transporter or SERT
2. Actions at several G-protein linked receptors (agonist actions at 5HT1A receptors, partial agonist actions at 5HT1B receptors, antagonist actions at 5HT1D and 5HT7 receptors)
3. Inhibition of a ligand-gated ion channel (the 5HT3 receptor)

Figure 1 Icon of vortioxetine showings its 6 pharmacologic mechanisms. Highlighted here is 5HT3 antagonism, linked to enhanced release of serotonin (5HT), NE, and ACh.
We have previously described the mechanisms whereby vortioxetine’s actions at 5HT receptors work together to enhance the release of 5HTReference Stahl5 and glutamateReference Stahl6 and to inhibit the release of GABA (gamma amino butyric acid).Reference Stahl6 Here we discuss how antagonism of 5HT3 receptors by vortioxetine (Figure 1) is one of the hypothetical mechanisms that also leads to enhanced release of 5HT as well as to enhanced release of NE and ACh.Reference Pehrson, Cremers and Bétry3, Reference Sanchez, Asin and Artigas4, Reference Stahl7, Reference Mørk, Montezinho and Miller8 An explanation of vortioxetine’s hypothetical actions at additional 5HT receptors that also contribute to the enhanced release of ease of NE and ACh, as well as to the release of DA and histamine HA, has been discussed elsewhere.Reference Pehrson, Cremers and Bétry3, Reference Sanchez, Asin and Artigas4, Reference Stahl7, Reference Mørk, Montezinho and Miller8
Serotonin and Glutamate Regulate Each Other
It is well known that 5HT regulates its own release via feedback mediated by autoreceptors that are located on the 5HT neurons themselves.Reference Stahl5, Reference Stahl9, Reference Fink and Gothert10 However, 5HT can also regulate its own release via postsynaptic 5HT3 receptors located within a 3-neuron feedback loop (Figure 2A).Reference Fink and Gothert10–Reference Ashby, Minabe, Edwards and Wang15 The first neuron in this feedback loop is serotonergic, and provides input to prefrontal cortex and hippocampus. There, 5HT stimulates excitatory 5HT3 receptors on a GABAergic interneuron (Figure 2A). This second neuron in the feedback loop is a type of GABAergic interneuron that does not stain positively for the calcium binding protein parvalbumin and is regular spiking, late spiking, or bursting in its firing pattern.Reference Stahl6, Reference Fink and Gothert10–Reference Gottlieb and Keller28 The GABA that is released from this second neuron in turn inhibits the third neuron in the feedback loop, namely, a pyramidal neuron in the prefrontal cortex or hippocampus (Figure 2A). This pyramidal neuron finally projects back to the midbrain raphe where it can release glutamate and stimulate 5HT release (Figure 2A). Thus, 5HT regulates downstream glutamate release, which in turn regulates 5HT release (Figure 2A).

Figure 2A Serotonin and glutamate regulate each other: role of 5HT3 receptors. Shown here is a 3-neuron feedback circuit, beginning with the 5HT neuron, terminating upon a 5HT3 receptor localized upon a second neuron: a GABAergic interneuron that does not stain positively for the calcium binding protein parvalbumin and has a firing pattern that is regular spiking, late spiking or bursting. GABA released from this second neuron in turn inhibits the third neuron in this feedback circuit: cortical pyramidal neurons that release glutamate at nerve terminals that project back to the midbrain raphe and that stimulate 5HT release.
Although SSRIs increase 5HT levels by SERT inhibition, activation of 5HT3 receptors by 5HT in this feedback loop leads to inhibition of cortical pyramidal neurons due to activation of GABAergic inhibition, and thus no amplification of 5HT release by downstream glutamate (Figure 2B).Reference Fink and Gothert10–Reference Ashby, Minabe, Edwards and Wang15 Contrast this with vortioxetine, which not only enhances 5HT via SERT inhibition, but also blocks 5HT3 receptors (Figure 2C). 5HT3 receptor antagonism removes GABAergic inhibition and thus disinhibits pyramidal neurons, further enhancing 5HT release by glutamatergic stimulation of serotonergic neurons in the midbrain raphe (Figure 2C).Reference Pehrson, Cremers and Bétry3, Reference Sanchez, Asin and Artigas4, Reference Dale, Zhang and Leiser16, Reference Bétry, Pehrson and Etievant17

Figure 2B When 5HT levels increase after administration of an SSRI, the activation of 5HT3 receptors by 5HT leads to stimulation of GABA release; this in turn inhibits cortical pyramidal neurons, and thus there is no amplification of 5HT release by downstream glutamate.

Figure 2C In contrast to the actions of SSRIs shown in Figure 2B, shown here are the actions of vortioxetine, which not only enhance 5HT via SERT inhibition, but also block 5HT3 receptors. The blockade of 5HT3 receptors removes GABA inhibition and thus disinhibits pyramidal neurons. This in turn enhances downstream release of 5HT due to glutamatergic stimulation of serotonergic neurons in the midbrain raphe.
Serotonergic Regulation of Norepinephrine (NE) and Acetylcholine (ACh) Release
5HT regulates the downstream release of many neurotransmitters, not only 5HT itself, but also NE, ACh, DA, and HA.Reference Pehrson, Cremers and Bétry3, Reference Sanchez, Asin and Artigas4, Reference Stahl9, Reference Fink and Gothert10, Reference Artigas18, Reference Pehrson and Sanchez24 Stimulation of postsynaptic 5HT1A receptors increases cortical and hippocampal release of AChReference Izumi, Washizuka, Miura, Hiraga and Ikeda29, Reference Consolo, Ramponi, Ladinsky and Baldi30 and NE.Reference Suzuki, Matsuda, Asano, Somboonthum, Takuma and Baba31, Reference Suwabe, Kubota, Niwa, Kobayashi and Kanba32 Although the microanatomy is still being worked out, blockade of postsynaptic serotonergic heteroreceptors on presynaptic nerve terminals could theoretically be another mechanism whereby ACh, NE, DA, and HA release is enhanced.Reference Mørk, Montezinho and Miller8, Reference Fink and Gothert10 These various mechanisms are discussed elsewhere.Reference Pehrson, Cremers and Bétry3, Reference Sanchez, Asin and Artigas4, Reference Stahl7–Reference Fink and Gothert10 Here we discuss and illustrate how 5HT3 receptors regulate both NE and ACh release. Specifically, 5HT stimulation of 5HT3 receptors causes inhibitory output from GABAergic interneurons, and this inhibits the release of NE and ACh from presynaptic nerve terminals (Figure 3A).Reference Matsumoto, Yoshioka, Togashi, Tochihara, Ikeda and Saito19, Reference Yan20 Thus, when SSRIs elevate 5HT levels, this causes GABA to be released, which in turn inhibits both NE and ACh release (Figure 3B).Reference Matsumoto, Yoshioka, Togashi, Tochihara, Ikeda and Saito19, Reference Yan20 By contrast, vortioxetine blocks the 5HT3 receptor so that GABA is not released by 5HT, and therefore both NE and ACh are disinhibited—ie, their levels are enhanced (Figure 3C).Reference Bang-Andersen, Ruhland and Jorgensen2, Reference Mørk, Montezinho and Miller8
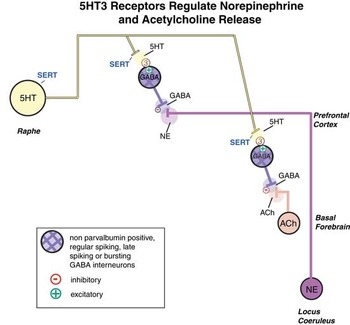
Figure 3A Serotonin (5HT) 3 receptor mediated regulation of ACh and NE release. 5HT stimulation of 5HT3 receptors causes inhibitory output from GABAergic interneurons, and this inhibits the release of NE and ACh from presynaptic nerve terminals.

Figure 3B SSRIs inhibit the release of ACh and NE via 5HT3 receptors. When SSRIs increase 5HT levels by SERT inhibition, GABA is released, which in turn inhibits both NE and ACh release.

Figure 3C Vortioxetine enhances the release of ACh and NE by blocking 5HT3 receptors. By contrast with SSRI actions shown in Figure 3B, vortioxetine blocks the 5HT3 receptor so that GABA is not released by 5HT, and therefore both NE and ACh are disinhibited—ie, their levels are enhanced.
Potential Clinical Significance of 5HT3 Antagonism
Enhanced release of 5HT by combining 5HT3 antagonism with SERT inhibition likely contributes to the antidepressant actions of vortioxetine.Reference Mørk, Pehrson and Brennum1–Reference Sanchez, Asin and Artigas4, Reference Mørk, Montezinho and Miller8, Reference Pehrson and Sanchez24 Enhanced 5HT release by combining SERT inhibition with several of vortioxetine’s other actions at 5HT receptors—namely 5HT1A agonism, 5HT1B partial agonism, 5HT1D antagonism, and 5HT7 antagonism—was discussed in a previous Brainstorms article.Reference Stahl5 Enhanced release of NE and ACh levels is also likely an important feature of vortioxetine’s clinical activity. That is, NE and ACh levels are increased with selective 5HT3 antagonists that also exhibit procognitive activity in animal models.Reference Brambilla, Ghiorzi, Pitsikas and Borsini33–Reference Arnsten, Line, Van Dyck and Stanhope37 Thus, vortioxetine’s procognitive actions in both animal modelsReference Pehrson and Sanchez24, Reference du Jardin, Jensen, Sanchez and Pehrson38–Reference Wallace, Pehrson, Sánchez and Morilak41 and in patients with major depressive disorderReference Katona, Hansen and Olsen42–Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe44 may be mediated in part by its 5HT3 antagonist properties and the enhanced release of both NE and ACh that accompany 5HT3 receptor antagonism. Adding 5HT3 antagonism to SSRI actions could potentially enhance not only the antidepressant actions of SSRI activity but also add procognitive actions to antidepressant actions via increased release of 5HT, ACh, and NE. This possibility fits with the notion that neurotransmitters may theoretically “tune” the malfunctioning brain circuits that cause psychiatric symptoms.Reference Stahl6, Reference Dale, Zhang and Leiser16, Reference Insel, Cuthbert and Garvey45, Reference Stahl46 The release of 5HT, NE, and ACh by vortioxetine could theoretically improve the efficiency of information processing in maladaptive brain circuits by facilitating long-term potentiation, synaptic plasticity, and enhanced pyramidal neuron activity leading to improvement not only of mood but also of cognitive symptoms in major depressive disorder.









