Introduction
The ability to detect, monitor, and respond to errors and mistakes enables us to maintain optimal performance across various tasks and situations (Olvet & Hajcak, Reference Olvet and Hajcak2008). Alterations in these processes are related to negative outcomes such as elevated anxiety (Moser et al., Reference Moser, Moran, Schroder, Donnellan, Yeung, Shackman, Cohen, Fox, Cavanagh, Hajcak and Orr2013). Researchers have leveraged event-related potentials (ERP) to investigate the neural bases of error processing and have identified the error-related negativity (ERN) as an ERP index of error monitoring processes. The ERN is an early fronto-central negative deflection that peaks between 0 and 150 ms following an erroneous response (e.g., in a Go/No-Go task; Gehring et al., Reference Gehring, Goss, Coles, Meyer and Donchin1993). The ERN is thought to underlie performance monitoring processes, indicating increased compensatory efforts once an error is committed on a task. During the transition to adolescence, youths undergo important developmental maturation in performance monitoring, characterized by an increase in the ERN amplitude with age (Tamnes et al., Reference Tamnes, Walhovd, Torstveit, Sells and Fjell2013). For instance, among 8- to 16-year-olds, older youths exhibited a larger ERN following errors on a task relative to their younger peers (Hajcak et al., Reference Hajcak, Franklin, Foa and Simons2008).
Inter-individual differences in the ERN have been linked to heightened anxiety symptoms in healthy (Bress et al., Reference Bress, Meyer and Hajcak2015; Weinberg et al., Reference Weinberg, Meyer, Hale-Rude, Perlman, Kotov, Klein and Hajcak2016) and clinically anxious youths (Hajcak et al., Reference Hajcak, Franklin, Foa and Simons2008; Meyer et al., Reference Meyer, Hajcak, Glenn, Kujawa and Klein2017). Among 8- to 18-year-old youths, an enlarged ERN was associated with greater anxiety symptoms (Filippi et al., Reference Filippi, Subar, Sachs, Kircanski, Buzzell, Pagliaccio, Abend, Fox, Leibenluft and Pine2020; McDermott et al., Reference McDermott, Perez-Edgar, Henderson, Chronis-Tuscano, Pine and Fox2009; Meyer, Reference Meyer2022). However, the ERN may be differentially associated with anxiety in younger children (Lawler et al., Reference Lawler, Hruschak, Aho, Liu, Ip, Lajiness‐O’Neill, Rosenblum, Muzik and Fitzgerald2021; Moser, Reference Moser2017): in 5- to 8-year-old children, a smaller ERN was found to be associated with greater temperamental fear or heightened anxiety symptoms (Lo et al., Reference Lo, Schroder, Fisher, Durbin, Fitzgerald, Danovitch and Moser2016; Torpey et al., Reference Torpey, Hajcak, Kim, Kujawa, Dyson, Olino and Klein2013). These age-specific patterns of the ERN-symptom association suggest that younger children may be more sensitive toward external (vs. internal) fear stimuli, whereas older youths become more responsive toward internal fear stimuli, for example, committing an error on a task (Meyer et al., Reference Meyer, Hajcak, Glenn, Kujawa and Klein2017; Weinberg et al., Reference Weinberg, Meyer, Hale-Rude, Perlman, Kotov, Klein and Hajcak2016).
While studies thus far have supported the developmental changes in the ERN and its relationship with anxiety during development (Meyer, Reference Meyer2017; Tamnes et al., Reference Tamnes, Walhovd, Torstveit, Sells and Fjell2013), the literature has exclusively focused on the inter-individual differences in the ERN by averaging the ERP signals across all error trials for each participant. Although such a signal averaging approach can increase the signal-to-noise ratio of the ERP data (Luck, Reference Luck2014), this approach assumes that the trial-to-trial signal during a given task is constant and that any within-person, trial-level variations are sources of noise. However, a small but growing literature, including our recent work (Liu et al., Reference Liu, Yang and Tan2024), has countered this assumption by evincing meaningful within-person, trial-level variations in the ERP data elicited by various paradigms (Volpert-Esmond et al., Reference Volpert-Esmond, Merkle, Levsen, Ito and Bartholow2018, Reference Volpert-Esmond, Page-Gould and Bartholow2021; Volpert-Esmond & Bartholow, Reference Volpert-Esmond and Bartholow2021; Von Gunten et al., Reference Von Gunten, Volpert-Esmond and Bartholow2018). By leveraging a multilevel modeling approach in examining the trial-level ERP data, these studies disentangled the between- and within-person variability and shed light on the dynamic patterns of information processing that unfolded over the course of a task.
Within this literature, only two studies have looked at the intra-individual (e.g., within-person, trial level) differences in the ERN in adults (Tobias & Ito, Reference Tobias and Ito2021; Volpert-Esmond et al., Reference Volpert-Esmond, Merkle, Levsen, Ito and Bartholow2018). One study found a linear decrease in the ERN amplitude (i.e., the ERN became more positive) as participants committed more errors during a speeded reaction task (Volpert-Esmond et al., Reference Volpert-Esmond, Merkle, Levsen, Ito and Bartholow2018). Researchers posited that this attenuation in the ERN might reflect decreased error salience over time related to participants’ adjustments of performance expectations as they made more errors on the task. Another study found that such attenuation in the ERN amplitude was moderated by individual differences in anxiety symptoms: ERN responses from the first to the second task repetition declined for women with lower anxiety symptoms but increased for women with higher anxiety symptoms (Tobias & Ito, Reference Tobias and Ito2021). In this case, more anxious individuals showed heightened, persistent monitoring of errors across repetitions of the task, potentially indicating a more “alerted” performance monitoring system relative to their less anxious peers. Collectively, these findings suggested that trial-to-trial variations in the ERN over the course of a task reflected meaningful patterns of performance monitoring that were associated with between-person differences.
However, no work has examined the within-person, trial-level differences in the ERN amplitude and their associations with age and anxiety symptoms during early adolescence, a period characterized by important developmental changes in error processing and heightened anxiety symptoms. To address this gap, we investigated the trial-level ERN data collected from 115 9- to 12-year-old community-dwelling youths during a Go/No-Go task. Specifically, we employed a multilevel modeling approach to examine the within-person changes in the ERN amplitude as youths committed more errors on the Go/No-Go task and how these within-person changes were associated with age and anxiety symptoms. Based on previous findings in adults that showed a decrease in the ERN following repeated errors on a task (Volpert-Esmond et al., Reference Volpert-Esmond, Merkle, Levsen, Ito and Bartholow2018), we expected that older youths, compared to younger youths, might show a more similar pattern to what was found in adults (i.e., declines in the ERN throughout the task). Previous work also observed declines in the ERN in less anxious women across task repetitions but increases in the ERN in more anxious women (Tobias & Ito, Reference Tobias and Ito2021). We therefore hypothesized that youths with heightened anxiety symptoms would similarly show increases (or smaller decreases) in the ERN over the course of the Go/No-Go task relative to their less anxious peers. Additionally, considering the age-related differences in the ERN-anxiety relationship during development (Meyer, Reference Meyer2017), we further explored the extent to which age interacted with anxiety symptoms in modulating the trial-to-trial changes in the ERN during the task.
Method
Participants and procedure
Data were drawn from an ongoing study investigating the neural correlates of cognitive risks for anxiety and depression in early adolescence. A community sample of 115 9- to 12-year-old youths (66 females, Mean age = 11.00 years, SD = 1.16) and their caregivers were recruited from a Midwestern urban area. None of the youths reported any major lifetime or present medical conditions or neurodevelopmental disabilities. Our study demographics were relatively representative of the local community (87.5% White, 3.6% Asian, 8.9% More than one race; 7.2% Hispanic or Latino). Family annual income ranged between $15,000 to $350,000 (Median = $95,000, 85% fall below $150,000).
Youths and their caregivers were invited to campus for a two-hour laboratory visit. Following caregiver consent and child assent, youths completed a battery of four EEG tasks (in counter-balanced order, including the Go/No-Go task) and an eye-tracking task tapping into different cognitive risk processes. Only data from the Go/No-Go task are reported here. After the lab visit, youths filled out an online questionnaire package reporting their behaviors and symptoms using the Qualtrics platform at home. Participants received monetary compensation for their participation. The study procedure was approved by the university’s Institutional Review Board.
Questionnaires
Youth self-report anxiety
The Youth Self-Report (YSR) is a 118-item checklist that assesses behavioral and emotional symptoms in 4- to 18-year-olds (Achenbach, Reference Achenbach1991). The YSR consists of a 16-item anxiety subscale (Kendall et al., Reference Kendall, Puliafico, Barmish, Choudhury, Henin and Treadwell2007), which was used in the current study to assess youths’ anxiety symptoms in the past month. Each item (e.g., I worry a lot) was rated on a 3-point scale (0 = not true, 2 = very true). A total score was computed to indicate anxiety symptoms. The YSR anxiety scale demonstrated good internal consistency in this study (Cronbach’s α = 0.87).
Children’s depression inventory
The 27-item child self-report version of the Children’s Depression Inventory (CDI; Kovacs, Reference Kovacs1978) assessed the presence and severity of depressive symptoms in 7- to 17-year-old youths. Due to limited inter-individual variability, we excluded the item “I want to kill myself.” For each of the remaining 26 items, youths were asked to select one of three statements that best described them for the past two weeks (e.g., I am sad… 0 = once in a while, 1 = many times, or 2 = all the time). A total score was calculated to indicate depressive symptom severity (Cronbach’s α in our current sample = .91).
The EEG Go/No-Go task
We adopted a youth-friendly version of the Go/No-Go task in combination with EEG recordings to elicit the ERN (Grammer et al., Reference Grammer, Carrasco, Gehring and Morrison2014; Ip et al., Reference Ip, Liu, Moser, Mannella, Hruschak, Bilek, Muzik, Rosenblum and Fitzgerald2019). The Go/No-Go task consisted of 252 Go trials and 63 No-Go trials divided into 3 blocks (84 Go and 21 No-Go trials per block, presented in a random order) with a self-paced break after the first block. As shown in Figure 1, each trial started with a fixation cross that lasted for 200 ms. Next, a black-and-white image of a dog or a cat was presented for 300 ms. Participants were instructed to press a button as quickly as possible when they saw a cat (Go) and not to press the button when they saw a dog (No-Go). The task proceeded to the next trial upon participants’ response; in the case of no response, a fixation appeared for 900 ms before moving on to the next trial. Participants took approximately 6 min to complete the task. The task was conducted using the E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA).

Figure 1. Trial procedure of the Go/No-Go task. Note. ms = milliseconds.
EEG data acquisition and processing
Youths completed the Go/No-Go task in an electrically shielded chamber, while continuous EEG signals were recorded using a 64-channel HydroCel Geodesic Sensor Net (Electrical Geodesic Inc.) and an EGI 200 NetAmps Amplifier. The EEG signals were recorded with a sampling rate of 250 Hz, referenced to the vertex electrode (Cz). Electrode impedances were kept below 50 kΩ. The EEG data were processed using Net Station Tools (Electrical Geodesics Inc., Eugene, OR). The raw EEG data were first filtered within the 0.1–40 Hz bandpass and re-referenced to the average of the two mastoid electrodes. Next, the data were time-locked to the response (i.e., button press) and segmented into desired epochs (100 ms pre-response to 500 ms following response), with a 100 ms baseline correction. Artifact detection was then conducted using default minimum-maximum parameters: a threshold (1) >200 μV for bad channels, (2) >140 μV for eye blinks, and (3) >50 μV for eye movements. We rejected segments with more than 30% bad channels, an eye movement, or an eye blink. Ninety-nine percent of the segments across all participants were retained. Finally, for each individual, we computed the mean amplitude of the 0–200 ms time window following each error across seven fronto-central channels (AFz, F1, Fz, F2, FC1, FCz, FC2) as the index of the trial-level ERN for subsequent analyses. The single-trial amplitude of the correct trials was not included in our analyses. Figure 2 illustrates the grand average ERP waveforms across the error and correct trials at the FC1 channel, where the ERN was maximal.

Figure 2. Grand average ERP waveforms and topographic maps of the ERN component at FC1 for correct and error trials. Note. ERN = error-related negativity; µV = microvolts; ms = milliseconds; topographic maps were generated as the mean activity across 0–200 ms. As expected, there was a negative deflection during the 0–200 ms time window in the error trials (mean/SD = −1.30/5.94 µV) compared to the correct trials (mean/SD = 3.86/3.01 µV, t(113) = 10.11, p < .001) across the seven fronto-central channels (AFz, F1, Fz, F2, FC1, FCz, FC2).
Multilevel growth analyses
To examine how the ERN varied as a function of error sequence (i.e., repeated errors committed over the course of the task), we first numbered all error trials sequentially for each participant (i.e., the first error was labeled as error number one, regardless of which trial it occurred on). We conducted stepwise multilevel growth modeling on the numbered trial-level ERN data to examine the effect of error sequence on the ERN amplitude and to what extent age and anxiety symptoms predicted the intercept and slope of the ERN across error trials. The intraclass correlation (.10) based on an empty multilevel model indicated that approximately 90% of the variance in the ERN was due to within-person, trial-level differences.
In Model 1, we treated error sequence as the within-person predictor of the intercept and the slopes of the ERN. Error sequence was baseline-centered to the first error on the task. Random effects were included for the intercept and slope of the ERN. The intercept represented the ERN at baseline; the slope represented the trial-to-trial changes over time in the ERN amplitude as more errors were committed. In Model 2, we maintained the same fixed predictors and random effects structure from Model 1 and added age and anxiety as between-person predictors of the baseline intercept and trial-to-trial changes of the ERN. Finally, in Model 3, we added the interaction term between age and anxiety. In Models 2 and 3, we also included sex, depressive symptoms, and total number of errors as between-person covariates. The between-person predictors in Models 2 and 3 were grand-mean centered. All models were estimated using the nlme library in R Studio (Pinheiro et al., Reference Pinheiro and Bates2023), with missing data handled using full-information maximum likelihood estimation.
Based on patterns of our trial-level ERN data, we included both linear and quadratic (i.e., curvilinear) slopes in our multilevel growth models. A chi-square test indicated that the quadratic model fitted significantly better with our data than the linear model (χ 2 (4) = 20.00, p = .001). Following the recommendations of Cohen et al. (Reference Cohen, Cohen, West and Aiken2003), we conducted post hoc simple slope analyses to probe the significant main effects and interactions on the quadratic changes of the ERN. Specifically, we examined the linear trends at different points of the error sequence (i.e., at error number 10, 20, and 30 representing the initial, middle, and later stages, respectively) at different levels of the between-person predictors.
Equations of the quadratic models are as follows:
Model 1:
 $$\small{\eqalign{ & Level\ 1\!:ERN_{ij} = {{\rm\beta}_{0j}} + {{\rm\beta}_{1j}}(error\ sequenc{e_{ij}}) + {{\rm\beta}_{2j}}{(error{{\ }}sequenc{e_{ij}})^2} +{e_{ij}} \cr & Level\ 2\!:{{\rm\beta}_{0j}} = {{\rm\gamma}_{00}} + {U_{0j}} \cr & \hskip34pt {{\rm\beta}_{1j}} = {{\rm\gamma}_{10}} + {U_{1j}} \cr & \hskip34pt {{\rm\beta}_{2j}} = {{\rm\gamma}_{20}} + {U_{2j}}}}$$
$$\small{\eqalign{ & Level\ 1\!:ERN_{ij} = {{\rm\beta}_{0j}} + {{\rm\beta}_{1j}}(error\ sequenc{e_{ij}}) + {{\rm\beta}_{2j}}{(error{{\ }}sequenc{e_{ij}})^2} +{e_{ij}} \cr & Level\ 2\!:{{\rm\beta}_{0j}} = {{\rm\gamma}_{00}} + {U_{0j}} \cr & \hskip34pt {{\rm\beta}_{1j}} = {{\rm\gamma}_{10}} + {U_{1j}} \cr & \hskip34pt {{\rm\beta}_{2j}} = {{\rm\gamma}_{20}} + {U_{2j}}}}$$
Model 2:
 $$\small{\eqalign{ & Level{\ }1\!:{{ }}ER{N_{ij}} = {{\rm\beta}_{0j}} + {{\rm\beta}_{1j}}(error{{\ }}sequenc{e_{ij}}){{ }} + {{\rm\beta}_{2j}}{(error{{\ }}sequenc{e_{ij}})^2} + \;{e_{ij}} \cr & Level{\ }2\!:{{\rm\beta}_{0j}} = {{\rm\gamma}_{00}} + {{\rm\gamma}_{01}}(SE{X_{.j}}) + {{\rm\gamma}_{02}}(nERROR{S_{.j}}) + {{\rm\gamma}_{03}}(DEPRES{S_{.j}}) + \cr & \hskip24pt \hskip36pt{{\rm\gamma}_{04}}(AG{E_{.j}}){{ }} + {{\rm\gamma}_{05}}(AN{X_{.j}}){{ }} + {{ }}{U_{0j}} \cr & \hskip37pt{{\rm\beta}_{1j}} = {{\rm\gamma}_{10}} + {{\rm\gamma}_{11}}(SE{X_{.j}}){{ }} + {{\rm\gamma}_{12}}(nERROR{S_{.j}}) + {{\rm\gamma}_{13}}(DEPRES{S_{.j}}){{ }} + \cr & \hskip61pt{{\rm\gamma}_{14}}(AG{E_{.j}}){m{ }} + {{\rm \gamma}_{15}}(AN{X_{.j}}){{ }} + {{ }}{U_{1j}} \cr & \hskip34pt \;{{\rm \beta}_{2j}} = {{\rm \gamma}_{20}} + {{\rm \gamma}_{21}}(SE{X_{.j}}) + {{\rm \gamma}_{22}}(nERROR{S_{.j}}) + {{\rm \gamma}_{23}}(DEPRES{S_{.j}}){{ }} + \cr & \hskip61pt {{\rm \gamma}_{24}}(AG{E_{.j}}){{ }} + {{\rm \gamma}_{25}}(AN{X_{.j}}){{ }} + {{ }}{U_{2j}} } }$$
$$\small{\eqalign{ & Level{\ }1\!:{{ }}ER{N_{ij}} = {{\rm\beta}_{0j}} + {{\rm\beta}_{1j}}(error{{\ }}sequenc{e_{ij}}){{ }} + {{\rm\beta}_{2j}}{(error{{\ }}sequenc{e_{ij}})^2} + \;{e_{ij}} \cr & Level{\ }2\!:{{\rm\beta}_{0j}} = {{\rm\gamma}_{00}} + {{\rm\gamma}_{01}}(SE{X_{.j}}) + {{\rm\gamma}_{02}}(nERROR{S_{.j}}) + {{\rm\gamma}_{03}}(DEPRES{S_{.j}}) + \cr & \hskip24pt \hskip36pt{{\rm\gamma}_{04}}(AG{E_{.j}}){{ }} + {{\rm\gamma}_{05}}(AN{X_{.j}}){{ }} + {{ }}{U_{0j}} \cr & \hskip37pt{{\rm\beta}_{1j}} = {{\rm\gamma}_{10}} + {{\rm\gamma}_{11}}(SE{X_{.j}}){{ }} + {{\rm\gamma}_{12}}(nERROR{S_{.j}}) + {{\rm\gamma}_{13}}(DEPRES{S_{.j}}){{ }} + \cr & \hskip61pt{{\rm\gamma}_{14}}(AG{E_{.j}}){m{ }} + {{\rm \gamma}_{15}}(AN{X_{.j}}){{ }} + {{ }}{U_{1j}} \cr & \hskip34pt \;{{\rm \beta}_{2j}} = {{\rm \gamma}_{20}} + {{\rm \gamma}_{21}}(SE{X_{.j}}) + {{\rm \gamma}_{22}}(nERROR{S_{.j}}) + {{\rm \gamma}_{23}}(DEPRES{S_{.j}}){{ }} + \cr & \hskip61pt {{\rm \gamma}_{24}}(AG{E_{.j}}){{ }} + {{\rm \gamma}_{25}}(AN{X_{.j}}){{ }} + {{ }}{U_{2j}} } }$$
Model 3:
 $$\small{\eqalign{ & Level{\rm{\ }}1\!:{\rm{ }}ER{N_{ij}} = {{\rm \beta}_{0j}} + {{\rm \beta}_{1j}}(error{\rm{\ }}sequenc{e_{ij}}){\rm{ }} + {{\rm \beta}_{2j}}{(error{\rm{\ }}sequenc{e_{ij}})^2} + \;{e_{ij}} \cr & Level{\rm{\ }}2\!:{{\rm \beta}_{0j}} = {{\rm \gamma}_{00}}_{} + {{\rm \gamma}_{01}}(SE{X_{.j}}){\rm{ }} + {{\rm \gamma}_{02}}(nERROR{S_{.j}}){\rm{ }} + {g_{03}}(DEPRES{S_{.j}}){\rm{ }} + \cr & \hskip60pt {{\rm \gamma}_{04}}(AG{E_{.j}}){\rm{ }} + {g_{05}}(AN{X_{.j}}){\rm{ }} + {{\rm \gamma}_{06}}(AN{X_{.j}})(AG{E_{.j}}){\rm{ }} + {\rm{ }}{U_{0j}} \cr & \hskip36pt {{\rm \beta}_{1j}} = {{\rm \gamma}_{10}} + {{\rm \gamma}_{11}}(SE{X_{.j}}){\rm{ }} + {{\rm \gamma}_{12}}(nERROR{S_{.j}}){\rm{ }} + {{\rm \gamma}_{13}}(DEPRES{S_{.j}}){\rm{ }} + \cr & \hskip60pt {{\rm \gamma}_{14}}(AG{E_{.j}}){\rm{ }} + {{\rm \gamma}_{15}}(AN{X_{.j}}){\rm{ }} + {{\rm \gamma}_{16}}(AN{X_{.j}})(AG{E_{.j}}){\rm{ }} + {\rm{ }}{U_{1j}} \cr & \hskip33pt \;{{\rm \beta}_{2j}} = {{\rm \gamma}_{20}} + {{\rm \gamma}_{21}}(SE{X_{.j}}){\rm{ }} + {{\rm \gamma}_{22}}(nERROR{S_{.j}}){\rm{ }} + {{\rm \gamma}_{23}}(DEPRES{S_{.j}}){\rm{ }} + \cr & \hskip60pt{{\rm \gamma}_{24}}(AG{E_{.j}}){\rm{ }} + {{\rm \gamma}_{25}}(AN{X_{.j}}){\rm{ }} + {{\rm \gamma}_{26}}(AN{X_{.j}})(AG{E_{.j}}){\rm{ }} + {\rm{ }}{U_{2j}} }} $$
$$\small{\eqalign{ & Level{\rm{\ }}1\!:{\rm{ }}ER{N_{ij}} = {{\rm \beta}_{0j}} + {{\rm \beta}_{1j}}(error{\rm{\ }}sequenc{e_{ij}}){\rm{ }} + {{\rm \beta}_{2j}}{(error{\rm{\ }}sequenc{e_{ij}})^2} + \;{e_{ij}} \cr & Level{\rm{\ }}2\!:{{\rm \beta}_{0j}} = {{\rm \gamma}_{00}}_{} + {{\rm \gamma}_{01}}(SE{X_{.j}}){\rm{ }} + {{\rm \gamma}_{02}}(nERROR{S_{.j}}){\rm{ }} + {g_{03}}(DEPRES{S_{.j}}){\rm{ }} + \cr & \hskip60pt {{\rm \gamma}_{04}}(AG{E_{.j}}){\rm{ }} + {g_{05}}(AN{X_{.j}}){\rm{ }} + {{\rm \gamma}_{06}}(AN{X_{.j}})(AG{E_{.j}}){\rm{ }} + {\rm{ }}{U_{0j}} \cr & \hskip36pt {{\rm \beta}_{1j}} = {{\rm \gamma}_{10}} + {{\rm \gamma}_{11}}(SE{X_{.j}}){\rm{ }} + {{\rm \gamma}_{12}}(nERROR{S_{.j}}){\rm{ }} + {{\rm \gamma}_{13}}(DEPRES{S_{.j}}){\rm{ }} + \cr & \hskip60pt {{\rm \gamma}_{14}}(AG{E_{.j}}){\rm{ }} + {{\rm \gamma}_{15}}(AN{X_{.j}}){\rm{ }} + {{\rm \gamma}_{16}}(AN{X_{.j}})(AG{E_{.j}}){\rm{ }} + {\rm{ }}{U_{1j}} \cr & \hskip33pt \;{{\rm \beta}_{2j}} = {{\rm \gamma}_{20}} + {{\rm \gamma}_{21}}(SE{X_{.j}}){\rm{ }} + {{\rm \gamma}_{22}}(nERROR{S_{.j}}){\rm{ }} + {{\rm \gamma}_{23}}(DEPRES{S_{.j}}){\rm{ }} + \cr & \hskip60pt{{\rm \gamma}_{24}}(AG{E_{.j}}){\rm{ }} + {{\rm \gamma}_{25}}(AN{X_{.j}}){\rm{ }} + {{\rm \gamma}_{26}}(AN{X_{.j}})(AG{E_{.j}}){\rm{ }} + {\rm{ }}{U_{2j}} }} $$
Results
Between-person descriptives and bivariate correlations
Table 1 shows the means, standard deviations, and bivariate correlations of the study variables. Girls were slightly older than boys. Compared to boys, girls made a smaller number of errors during the task, showed a larger ERN, and reported higher symptoms of anxiety and depression. Anxiety symptoms were positively correlated with depressive symptoms.
Table 1. Mean, standard deviation, and bivariate correlations of study variables
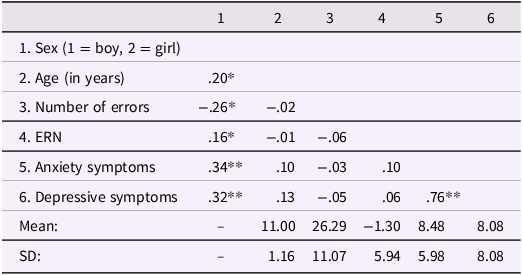
Note. *p < .05, **p < .01 (2-tailed). ERN = error-related negativity; SD = standard deviation; the ERN was averaged across trials.
Model 1: The intercept and changes of the ERN. Table 2 displays the results of the stepwise multilevel growth analyses. In the first model with baseline-centered error sequence (the first error trial) as the predictor, we found a significant negative linear effect of error sequence (γ = −0.375, SE = 0.101, t (2881) = −3.725, p < .001), indicating that the trial-level ERN amplitude increased (i.e., became more negative) at the beginning of the task (Cohen et al., Reference Cohen, Cohen, West and Aiken2003). We also found a significant positive quadratic effect of error sequence (γ = 0.008, SE = 0.003, t (2881) = 2.581, p = .010), indicating that after youths’ trial-level ERN increased earlier in the task, it subsequently decreased as more errors were committed later in the task (see Figure 3).Footnote 1

Figure 3. Predicted values of the quadratic slope of the ERN across error trials. Note. ERN = error-related negativity.
Table 2. Stepwise multilevel growth models predicting the baseline and trial-to-trial changes of the ERN amplitude

Note. *p < .05, **p < .01 (2-tailed). ERN = error-related negativity; Est. = estimate; SE = standard error.
Model 2: Age and anxiety predicting the baseline and trial-level changes of the ERN. In our subsequent model, we observed an effect of anxiety on the baseline (intercept) of the ERN (γ = 0.444, SE = 0.220, t (102) = 2.017, p = .046), with higher anxiety symptoms associated with a smaller, less negative ERN on the first error trial. Age significantly predicted quadratic trial-to-trial changes in the ERN (γ = 0.006, SE = 0.002, t (2706) = 2.534, p = .011). Decomposing the quadratic changes at different levels of age indicated that younger youths (mean−1SD = 9.84 years old) showed an increase in the ERN (i.e., became more negative) during the initial (at error number 10; γ = −0.215, SE = 0.066, p = .001) and middle (at error number 20; γ = −0.166, SE = 0.076, p = .028), but not the later (at error number 30; γ = −0.118, SE = 0.158, p = .456) stages of the task. Youths of mean age (11.01 years old) showed an increase in the ERN during the initial (at error number 10; γ = −0.245, SE = 0.048, p < .001), but not the middle (at error number 20; γ = −0.062, SE = 0.066, p = .353) or later (at error number 30; γ = 0.122, SE = 0.139, p = .381) stages of the task. Youths of older age (mean + 1SD = 12.18 years old), on the other hand, showed an increase in the ERN during the initial (at error number 10; γ = −0.275, SE = 0.069, p < .001) but not the middle (at error number 20; γ = 0.043, SE = 0.079, p = .582) stages, and a subsequent decline in the ERN (i.e., became less negative or more positive) during the later stage (at error number 30; γ = 0.362, SE = 0.164, p = .028; see Figure 4).

Figure 4. Predicted values of the effect of age on the quadratic slope of the ERN across error trials. Note. ERN = error-related negativity; SD = standard deviation.
Model 3: Interaction between age and anxiety in predicting the baseline and trial-level changes of the ERN. In the final model, we found a significant age by anxiety interaction on the trial-to-trial quadratic changes in the ERN (γ = 0.001, SE = 0.000, t (2704) = 2.231, p = .026). We then probed the interaction by evaluating rates of quadratic changes in the ERN at different levels of age and anxiety symptoms (Figure 5). For the younger age group (mean−1SD = 9.84 years old), youths with higher anxiety (mean + 1SD) showed increases in the ERN (i.e., became more negative) during the initial (at error number 10; γ = −0.353, SE = 0.115, p = .002), middle (at error number 20; γ = −0.449, SE = 0.100, p < .001), and later (at error number 30; γ = −0.546, SE = 0.219, p = .013) stages of the task. Youths with mean anxiety showed increases in the ERN during the initial (at error number 10; γ = −0.212, SE = 0.065, p = .001) and middle (at error number 20; γ = −0.151, SE = 0.066, p = .022) stages, but not the later (at error number 30; γ = −0.090, SE = 0.140, p = .521) stage of the task. Youths with lower anxiety (mean−1SD) did not show any significant changes in the ERN throughout the task, except a marginal declining pattern during the later stage (at error number 30; γ = 0.367, SE = 0.193, p = .058).

Figure 5. Predicted values of the interaction between age and anxiety on the quadratic slope of the ERN across error trials. Note. ERN = error-related negativity; SD = standard deviation.
For the mean age group (11.01 years old), those with higher anxiety exhibited increases in the ERN during the initial (at error number 10; γ = −0.412, SE = 0.085, p < .001) and the middle (at error number 20; γ = −0.230, SE = 0.081, p = .004) stages, but not the later (at error number 30; γ = −0.049, SE = 0.168, p = .773) stage of the task. Youths with mean anxiety exhibited increases in the ERN during the initial stage (at error number 10; γ = −0.242, SE = 0.047, p < .001), but not the middle (at error number 20; γ = −0.041, SE = 0.061, p = .502) or later (at error number 30; γ = 0.160, SE = 0.130, p = .219) stages. Youths with lower anxiety did not show any significant changes in the ERN during the early (at error number 10; γ = −0.071, SE = 0.083, p = .393) and middle (at error number 20; γ = −0.149, SE = 0.079, p = .059) stages, but a decrease in the ERN in the later stage (at error number 30; γ = 0.368, SE = 0.168, p = .029).
For the older age group (mean + 1SD = 12.18 years old), those with higher anxiety experienced increases in the ERN during the initial (at error number 10; γ = −0.471, SE = 0.107, p < .001), but not the middle (at error number 20; γ = −0.011, SE = 0.100, p = .911) stages, and a subsequent decline in the ERN in the later stage (at error number 30; γ = 0.449, SE = 0.206, p = .030). Similarly, those with mean anxiety showed increases in the ERN during the initial (at error number 10; γ = −0.271, SE = 0.067, p < .001), but not the middle (at error number 20; γ = 0.069, SE = 0.071, p = .328) stages, and a subsequent decline in the ERN in the later stage (at error number 30; γ = 0.409, SE = 0.148, p = .006). Youths with lower anxiety showed no significant changes in the ERN except a marginal decline during the later stage of the task (at error number 30; γ = 0.370, SE = 0.194, p = .057). These patterns indicated that the effect of anxiety on trial-level changes in the ERN was different for younger and older youths. Higher anxiety was associated with an increasing pattern in the ERN across early errors on the task in all age groups, but a decline in the ERN across later errors only in older youths. In contrast, lower anxiety was not associated with as much trial-level changes in the ERN across different ages, except for a later decline in the mean age group.
Discussion
Existing developmental studies on error processing have focused on the between-person differences in the ERN and their associations with age and anxiety in youths. However, it remained unclear to what extent youths’ ERN varied at the within-person level over the course of a task and how these within-person variations were associated with their age and anxiety symptoms. Using a multilevel modeling approach, we for the first time examined the trial-to-trial changes in the ERN throughout a Go/No-Go task in a group of community-dwelling 9- to 12-year-olds and how age and anxiety symptoms modulated these trial-level changes. Our study provided novel evidence of the within-person, trial-level changes in the ERN over the course of a Go/No-Go task, which were further modulated by youths’ age and anxiety symptoms. These findings contributed to the mechanistic knowledge of error-related processes and their role in the development of psychopathology.
In our multilevel growth analyses, we observed a quadratic, curvilinear change in the ERN as more errors were committed during the Go/No-Go task. Specifically, youth showed a trial-to-trial increase in the ERN earlier in the task, followed by a gradual decline in the ERN as they made more errors later in the task. These trial-level changes were further modulated by age, such that the decline in ERN, following the initial increase, occurred only in older youths (of mean + 1 SD age). Younger youths showed a stable increase in the trial-level ERN over the earlier and middle stages of the task. These age-specific patterns of trial-level changes in the ERN extended previous findings in adults. In those findings, adults showed linear declines in the ERN during speeded reaction tasks (Tobias & Ito, Reference Tobias and Ito2021; Volpert-Esmond et al., Reference Volpert-Esmond, Merkle, Levsen, Ito and Bartholow2018), which were posited to reflect decreased error salience related to downward adjustments of error monitoring resources following repeated errors (Volpert-Esmond et al., Reference Volpert-Esmond, Merkle, Levsen, Ito and Bartholow2018). However, these studies did not investigate if there existed any curvilinear changes in adults’ ERN. By examining both the linear and curvilinear changes in the ERN in youths, our findings suggested that older, but not younger, youths might have engaged in upward adjustments first and devoted greater compensatory efforts toward repeated errors earlier in the task (reflected by the early increase in the ERN), before down-regulating their error responses in a more “adult-like” manner later in the task (reflected by a later decrease in the ERN).
The literature has documented the between-person differences in the developmental patterns of error processing as indexed by the ERN. Typically, the ERN amplitude increases (i.e., becomes more negative) with age as children transition into adolescence, indicating improved performance monitoring capacities (Tamnes et al., Reference Tamnes, Walhovd, Torstveit, Sells and Fjell2013). Extending this work, our study found age-specific patterns in the ERN on the within-person level in 9- to 12-year-olds, such that only those who were relatively older showed an initial increase followed by a subsequent decrease in the ERN. Together, previous research and our current findings point to distinct maturation patterns on the between-person and within-person levels of the ERN during development.
Contrary to our expectations based on the adult literature (Tobias & Ito, Reference Tobias and Ito2021), we did not observe a significant main effect of anxiety symptoms on the within-person changes in the ERN. Notably, the adult study that reported an effect of anxiety on the within-person changes in the ERN examined the mean differences of the ERN between two task repetitions, which might not have captured the trial-to-trial changes in the ERN (Tobias & Ito, Reference Tobias and Ito2021). However, we did find a significant interaction between anxiety and age in predicting the trial-level curvilinear changes in the ERN. For younger youths, those with higher anxiety showed a continuous increase in the ERN over the course of the task, indicating a more persistent and alerted error monitoring pattern throughout the task; younger youths with lower anxiety showed no changes in the ERN over time. In other words, while younger youths in general were less capable of down-regulating their error monitoring processes compared to their older peers (as indicated by the main effect of age), this capacity could be further hampered by heightened anxiety symptoms (Muris & Ollendick, Reference Muris and Ollendick2005; Scheper et al., Reference Scheper, Majdandžić, van de Ven, Jansen, Doreleijers, Schuengel and de Vries2017).
Older youths with higher anxiety also showed increases in the ERN following initial errors on the task. However, this initial increase was followed by a decline in the ERN with subsequent errors later in the task. In other words, older youths who were more anxious showed heightened responses toward errors early in the task but were able to gradually adjust down those responses over time. This suggested that while both younger and older youths with higher anxiety tended to show increases in the ERN early in the task, those who were older might have better, more flexible performance monitoring capacities that allowed them to down-regulate their responses to errors later in the task (Kadosh et al., Reference Kadosh, Heathcote and Lau2014).
As reviewed earlier, the literature has delineated the age-specific patterns of the association between anxiety and between-person differences in the ERN (Olvet & Hajcak, Reference Olvet and Hajcak2008): a larger ERN was associated with heightened anxiety in older youths (Meyer, Reference Meyer2022), whereas a smaller ERN was linked to higher anxiety in younger children (Filippi et al., Reference Filippi, Subar, Sachs, Kircanski, Buzzell, Pagliaccio, Abend, Fox, Leibenluft and Pine2020; McDermott et al., Reference McDermott, Perez-Edgar, Henderson, Chronis-Tuscano, Pine and Fox2009). Our recent work based on the same 9- to 12-year-old sample observed a stronger between-person ERN-anxiety association among older youths (Tan & Liu, Reference Tan and Liu2024). This developmental shift may reflect the maturation of the error monitoring system, characterized by a greater sensitivity toward internal fear such as mistakes made on a task (Lawler et al., Reference Lawler, Hruschak, Aho, Liu, Ip, Lajiness‐O’Neill, Rosenblum, Muzik and Fitzgerald2021). Extending this literature, we provided novel evidence on the age-specific patterns of the association between anxiety and within-person differences in the ERN during a task, such that the effect of anxiety on the within-person changes in the ERN was more evident in younger, and not older, youths.
Our study was the first to investigate the within-person, trial-level changes in the ERN throughout a Go/No-Go task in early adolescents. We also investigated to what extent age and anxiety symptoms modulated these trial-to-trial changes in the ERN. We took a dimensional approach by examining an unselected, low-risk community sample with emerging anxiety symptoms. Such an approach, compared to group comparisons (e.g., between clinical and non-clinical samples), increases statistical power and sheds light on the processes shared by typical and atypical development (Cicchetti & Toth, Reference Cicchetti, Toth, Nolen-Hoeksema and Hilt2009). However, our sample size was relatively small for examining a three-way interaction (i.e., the Age × Anxiety interaction on the quadratic slope of the ERN), warranting future research with larger sample sizes. Future research in high-risk or clinical samples with more severe anxiety symptoms is necessary to advance our understanding of the relationship between anxiety and within-person variations in the ERN. Further work in older adolescents and more ethnically diverse populations will also help determine whether the observed effects in this study are similarly present beyond early adolescence and White-dominant youth samples. Another limitation was the cross-sectional design that prevented us from making directional inferences between anxiety and the trial-level patterns of the ERN. For example, for younger youths with heightened anxiety who showed continuous increases in their trial-level ERN, it is unclear whether they were more anxious because they were less able to adjust their error responses during a task, or they were not able to adjust those error responses because they were more anxious. Longitudinal studies are warranted to answer these questions.
In sum, our study provided the first evidence of the within-person, curvilinear changes in the trial-level ERN in early adolescents. The ERN increased earlier in the task and subsequently declined as more errors were made later in the task. We also elucidated the effects of age and anxiety on the trial-level changes in the ERN. These findings suggested that the within-person, trial-level variations in the ERN reflected meaningful patterns of the dynamic error monitoring processes during a task and were associated with between-person differences such as age and anxiety symptoms. Our study contributed important insights into the development of ERN in youths and the underlying mechanisms of the ERN-anxiety relationship that cannot be captured by between-person approaches.
Data availability statement
The current study was not preregistered. Data are available upon request. Correspondence concerning this article should be addressed to Jaron Xe Yung Tan, University of Alberta, P217 Biological Sciences Building, 11,455 Saskatchewan Drive, Edmonton, Alberta, Canada T6G 2E9.
Funding statement
This study is supported by an NIGMS Centers of Biomedical Research Excellence (P30 GM114748, “COBRE Center for Visual and Cognitive Neuroscience”) pilot grant to Pan Liu.
Competing interests
The authors have no known conflict of interest to disclose.









