Introduction
By skewing information processing resources toward a negative (or positive) mental perspective, cognitive biases are instrumental to the development and maintenance of psychopathology (or wellbeing) (Beck et al., Reference Beck, Emery, Greenberg and Therapy1985; Williams et al., Reference Williams, Watts, MacLeod and Mathews1988, Reference Williams, Watts, MacLeod and Mathews1997). Although the protective and deleterious qualities of cognitive biases are well documented (e.g., Booth et al., Reference Booth, Songco, Parsons and Fox2022; Songco et al., Reference Songco, Booth, Spiegler, Parsons and Fox2020), less is known about what influences their development. Understanding how cognitive biases proliferate, particularly during key developmental periods like adolescence, could point toward effective prevention and intervention strategies that enhance their positive and diminish their negative effects.
The Cognitive Bias (CogBIAS) framework offers an interdisciplinary approach for exploring the development of cognitive biases (Fox & Beevers, Reference Fox and Beevers2016; Fox & Keers, Reference Fox and Keers2019). Integrating cognitive and genetic research, the CogBIAS model proposes that cognitive biases develop as a function of environmental qualities (which determine the valence of biases) and the genetic sensitivity to those qualities (which determines the potency of biases). A large body of research has established the role of adversity in the development of negative cognitive biases (Fani et al., Reference Fani, Bradley-Davino, Ressler and McClure-Tone2011; Pine et al., Reference Pine, Mogg, Bradley, Montgomery, Monk, McClure, Guyer, Ernst, Charney and Kaufman2005; Pollak & Kistler, Reference Pollak and Kistler2002; Pollak & Tolley-Schell, Reference Pollak and Tolley-Schell2003; Zavos et al., Reference Zavos, Gregory and Eley2012). While less research has explored genetic influences to their development, twin studies have established their genetic basis and overlap with internalizing conditions (Anokhin et al., Reference Anokhin, Golosheykin, Grant and Heath2011; Eley et al., Reference Eley, Gregory, Clark and Ehlers2007, Reference Eley, Gregory, Lau, McGuffin, Napolitano, Rijsdijk and Clark2008; Rijsdijk et al., Reference Rijsdijk, Riese, Tops, Snieder, Brouwer, Smid and Ormel2009). However, further molecular investigations have been limited and confined to studies of individual candidate genes. Such studies have focused on linking “popular” genetic variants (such as the serotonin transporter polymorphism) with “popular” cognitive biases (e.g., in the attention and interpretation of information) (Beevers et al., Reference Beevers, Gibb, McGeary and Miller2007, Reference Beevers, Wells, Ellis and McGeary2009; Fox et al., Reference Fox, Ridgewell and Ashwin2009; Fox & Standage, Reference Fox and Standage2012; Pergamin-Hight et al., Reference Pergamin-Hight, Bakermans-Kranenburg, van IJzendoorn and Bar-Haim2012). Similarly, studies investigating gene-by-environment interactions have focused mostly on candidate genes and their effects on risk for particular psychopathologies, but not their cognitive endophenotypes (e.g., Assary et al., Reference Assary, Vincent, Keers and Pluess2018; Caspi & Moffitt, Reference Caspi and Moffitt2006; Manuck & McCaffery, Reference Manuck and McCaffery2014).
Crucially, genetic research has thus far been guided by the diathesis-stress model (Monroe & Simons, Reference Monroe and Simons1991), focusing exclusively on genes implicated in negative outcomes. Over the last decade, however, researchers have expanded the diathesis-stress model by suggesting that some psychiatric genetic variants might confer sensitivity to positive environmental influences (i.e., vantage sensitivity) (Pluess & Belsky, Reference Pluess and Belsky2013), or to both positive and negative influences (i.e., differential sensitivity) (Belsky et al., Reference Belsky, Bakermans-Kranenburg and van IJzendoorn2007; Belsky, Reference Belsky1997). This broader model of “differential susceptibility” proposes that individuals are not merely “vulnerable” to negative life circumstances, but rather “susceptible” to both negative and positive life circumstances (see Belsky & Pluess, Reference Belsky and Pluess2009; Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn2011). In this sense, an individual is not only likely to wither under adverse conditions but is simultaneously likely to disproportionately prosper under enriching life conditions.
To our knowledge, only one study examined cognitive biases using such a general susceptibility approach. This study, by Fox et al. (Reference Fox, Zougkou, Ridgewell and Garner2011), supported the notion of differential susceptibility, by showcasing that individuals with a vulnerability to depression could be trained to develop a strong attentional bias to either positive or negative affective stimuli. However, much like other studies in this area, Fox et al.’s (Reference Fox, Zougkou, Ridgewell and Garner2011) investigation was severely limited, since its operationalization of “vulnerability” was confined to a single genetic variant (the serotonin transporter gene) (see Britton & Rauch, Reference Britton and Rauch2011 for commentary). In contrast, the genetic architecture of psychological phenotypes is increasingly recognized to be “polygenic,” meaning that it comprises hundreds of thousands of such variants (with each exerting a small effect on phenotypic outcomes) (see O’Donovan & Owen, Reference O’Donovan and Owen2016). Thus, composite measures that summarize the effects of many variants (such as “polygenic risk scores”) are considered better proxies of genetic “risk” (Ronald, Reference Ronald2020).
To date, though, only a few studies have used polygenic risk scores (PRSs) to examine psychological outcomes from the differential susceptibility perspective. The first polygenic study on the matter was conducted by Keers et al. (Reference Keers, Coleman, Lester, Roberts, Breen, Thastum, Bögels, Schneider, Heiervang, Meiser-Stedman, Nauta, Creswell, Thirlwall, Rapee, Hudson, Lewis, Plomin and Eley2016) and revealed that genetic sensitivity predicts greater response to cognitive behavioral therapy in children with anxiety disorders. A related study by Keers and Pluess (Reference Keers and Pluess2017) highlighted the importance of such sensitivity during early development. However, no further research has employed a similar polygenic approach to investigate intermediary phenotypes, such as cognitive biases, which could lie on the pathway between sensitivity and either psychopathology (e.g., Songco et al., Reference Songco, Booth, Spiegler, Parsons and Fox2020) or resilience (e.g., Booth et al., Reference Booth, Songco, Parsons and Fox2022).
In this study, we adopted such a polygenic approach to assess the development of memory and interpretation biases in an adolescent sample from the CogBIAS Longitudinal Study (CogBIAS-L-S) (Booth et al., Reference Booth, Songco, Parsons, Heathcote, Vincent, Keers and Fox2017). In keeping with evidence regarding the shared genetic architecture of cognitive biases and depression (see Vincent, Reference Vincent2019), we conceptualized genetic “risk” (or sensitivity) using a PRS for major depressive disorder (MDD). Our PRS was constructed using summary statistics from a recent genome-wide association study (GWAS) (Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui, Adams, Agerbo, Air, Andlauer, Bacanu, Bækvad-Hansen, Beekman, Bigdeli, Binder, Blackwood, Bryois, Buttenschøn, Bybjerg-Grauholm and Sullivan2018), which features better phenotyping of cases and controls than related GWAS that focus on broad or minimal phenotyping criteria (Cai et al., Reference Cai, Revez, Adams, Andlauer, Breen, Byrne, Clarke, Forstner, Grabe and Hamilton2020). Beyond polygenic scores, we also employed measures of life experiences. Using these measures, we examined the extent to which positive and negative life experiences, polygenic risk for depression, and their gene-by-environment (G × E) interaction influenced the development of cognitive biases across adolescence. We hypothesized that life experiences and polygenic risk for depression will be independently and interactively associated with cognitive biases across adolescence, and preregistered these hypotheses on the Open Science Framework (on which our code, some null results, and additional supplementary information is further available).
Methods
Sample
We used data from the CogBIAS-L-S (see Booth et al., Reference Booth, Songco, Parsons, Heathcote, Vincent, Keers and Fox2017 for a full description of measures and study protocol). The CogBIAS-L-S is a three-wave study that tracked the psychological development of 504 adolescents across South England, UK. The first assessment occurred during early secondary school (ages 12 to 14), with two follow-up assessments occurring at 12- to 18-month intervals. Dropout rates were low (11% at Wave 2, N = 450; 19% at Wave 3, N = 411) and largely due to school absences. While most participants were White Europeans (74.33%), the sample also included Asians (12.22%), Africans (2.67%), as well as individuals of mixed ancestry (6.16%) and others (3.49%). However, because our polygenic scores were derived from a GWAS of primarily European populations (which are not generalizable to individuals of other ancestry) (Roberts et al., Reference Roberts, Khoury and Mensah2019), our analyses were conducted only on the White European sample. Demographic characteristics of this sample (N = 337) are outlined at Table 1 (see also Table S1 and Booth et al., Reference Booth, Songco, Parsons, Heathcote and Fox2019).
Table 1. Demographic characteristics of cohort and descriptive statistics of measurement instruments

Ethical considerations
Ethical approval for the CogBIAS project was acquired from the National Research Ethics Service (NRES) of NHS (National Health Service) England. In particular, the NRES Committee South Central (14/SC/0128) approved the collection of genetic information and administration of psychological tests on the 30 September 2014 (Project ID: 141833). Informed consent was obtained in written format by parents and adolescents.
Procedure
Testing sessions were conducted either within participants’ schools or at the Department of Experimental Psychology, University of Oxford. Assessments consisted of two sessions (1 hour each), completed in a row or over separate days. Each session was completed in computer labs, under exam conditions. Prior to the assessment, participants were briefed on the study procedure, before written assent was collected (informing them that they could stop their participation, at any point). Parental consent was obtained in writing, prior to data-collection. In each session, participants completed a battery of measurement instruments (both self-report and behavioral). For this study, we used data from six measures (Table 1). (1) DNA samples were collected at wave 1. Self-reported measures of (2) life events, (3) anxiety, and (4) depression scores were collected at all waves. Behavioral assessments of (5) memory and (6) interpretation biases were also collected at all waves.
Genotyping
Saliva samples were collected with the use of DNA Genotek Oragene OG-500 collection kits. Genomic data were then extracted and stored at –80 °C, in accordance with established protocols. A total of 496 participants provided adequate DNA samples (200 mg). This sample was genotyped using the Illumina Human Omni express-24. Genome-wide data were subject to standard quality control using a well-established pipeline (Coleman et al., Reference Coleman, Euesden, Patel, Folarin, Newhouse and Breen2016). This included the removal of any duplicate single-nucleotide polymorphisms (SNPs), the exclusion of SNPs with minor allele frequencies (MAFs) <0.05, SNP missingness >0.01, and any deviating from Hardy–Weinberg equilibrium p < 1 × 10−8. Individuals were excluded due to gender mismatches; heterogeneity >3 standard deviations; individual missingness >0.01; and cryptic relatedness assessed as a proportion of identity by descent (IBD >0.1875). This resulted in the retention of 594,667 SNPs across 491 participants. An additional 5,129,755 SNPs were then imputed using the 1000 Genomes phase 3 reference panel (The 1000 Genomes Project Consortium, 2015). Further quality control in line with the Coleman, Euesden, et al., (Reference Coleman, Euesden, Patel, Folarin, Newhouse and Breen2016) protocol, was then conducted, excluding poorly imputed SNPs (INFO <0.3), those with a MAF <0.05, and SNP missingness <0.01. This resulted in a total of 5,596,260 genotyped and imputed SNPs remained for analysis (see Appendix S1). For the purpose of the current study, and for reasons previously highlighted, the sample was then reduced to include only individuals of European descent (N = 391).
Measures
Exogenous variables
Life events. Endorsed life experiences were measured using the Child Adolescent Survey of Experiences (CASE; Allen & Rapee, Reference Allen and Rapee2012). This questionnaire comprised 38 life experiences, relevant to the adolescent life (e.g., “I broke up with my boyfriend/girlfriend” or “I did well in an important test”). Participants were asked to report whether any of these events had occurred in their lives in the past 12 months; if so, they were then asked to rate that event on a 6-point Likert scale (1 = “Really bad”, 2 = “Quite bad”, 3 = “A little bad”, 4 = “A little good”, 5 = “Quite good”, 6 = “Really good”). An option to rate another two life experiences from their lives was also given. Unlike other measures, this scoring allows participants to choose the valence of each life event. Variables representing positive and negative life experiences were calculated using the total number of endorsed life events that were rated in a positive manner (i.e., ratings of 4, 5, or 6) and negative manner (i.e., ratings of 1, 2, or 3), respectively (see Table 1).
Polygenic scoring. The polygenic scores for depression were constructed using publicly available summary statistics from a GWAS meta-analysis of MDD (Wray et al., Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui, Adams, Agerbo, Air, Andlauer, Bacanu, Bækvad-Hansen, Beekman, Bigdeli, Binder, Blackwood, Bryois, Buttenschøn, Bybjerg-Grauholm and Sullivan2018), obtained from the psychiatric genomic consortium (http://pgc.unc.edu). The GWAS summary statistics were subject to standard quality control procedures, including the removal of duplicate and ambiguous SNPs, and the exclusion of any SNPs with a MAF <0.05 (see Appendix S1 for details). The PRSice v1.25 (Euesden et al., Reference Euesden, Lewis and O’Reilly2015) was then used to create nine PRS variables, at incremental p value thresholds: 0.001 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 1.00 (see Appendix S1 for number of SNPs in each threshold).
Depression and anxiety. To measure internalizing psychopathology, we used the Revised Children’s Anxiety and Depression Scale, Short Form (Ebesutani et al., Reference Ebesutani, Reise, Chorpita, Ale, Regan, Young, Higa-McMillan and Weisz2012), a psychometrically validated, self-report questionnaire that comprises 25 items relating to depression and anxiety (recorded on a 4-point Likert scale). Total scores, indicative of higher levels of depression and anxiety, were created by summing up the items of each scale.
Endogenous variables
Memory bias. The Self-Referential Encoding Task (SRET; see Booth et al., Reference Booth, Songco, Parsons, Heathcote and Fox2019) was used to measure memory bias. The SRET comprises three phases: encoding, distraction, and surprise recall. In the encoding phase, participants viewed 44 self-referent adjectives (half positive, half negative), which were displayed successively for 200 ms (each) on a monitor, answering with “yes” or “no” the query: “Describes me?”. In the distraction phase, participants were asked to solve three simple mathematical questions (e.g., “What is 2 × 3?”), the responses of which were irrelevant to the task. Finally, in the surprise-recall phase, participants were asked to type as many words as they could recall from the first phase within a 3-minute period. Three outcomes were calculated and used in our analyses: (1) the number of negative words recalled (“Negative Recall”), (2) the number of positive words recalled (“Positive Recall”), and (3) an overall Memory Bias score, computed as: (Negative Recall – Positive Recall)/(Total recall). (Positive scores on the Memory Bias indicate preferential processing of negative information; negative scores on the Memory Bias indicate preferential processing for positive information; finally, a score of “0” implies no bias at all.)
Interpretation bias. The Adolescent Interpretation and Belief Questionnaire (AIBQ; Miers et al., Reference Miers, Blöte, Bögels and Westenberg2008) was used to measure interpretation bias. Participants were asked to imagine themselves in 10 ambiguous scenarios (five social and five non-social), and rate how likely each of three possible interpretations (positive, negative, or neutral) was to “pop into their mind,” using a 5-point Likert scale (1 = “Doesn't pop up in my mind,” 3 = ”Might pop up in my mind,” 5 = “Definitely pops up in my mind”). Six outcome measures were calculated and used in our analyses. Four interpretation scores: (1) Negative Social, (2) Positive Social, (3) Negative Non-Social, and (4) Positive Non-Social Interpretations (based on the average of their respective items). And two bias scores: (5) Social Interpretation Bias (calculated as: Negative Social Interpretation – Positive Social Interpretation) and (6) Non-Social Interpretation Bias (calculated as: Negative Non-Social Interpretation – Positive Non-Social Interpretation), which reflected the degree of a bias for negative over positive interpretations, in the social and non-social domains, respectively.
Statistical analyses
Longitudinal modeling. To examine the longitudinal development of cognitive biases, we used two linear mixed effects models: for independent and interactive effects. In both models, we included random intercepts for participants and random slopes for time to account for the covariance of repeated measures (Fitzmaurice et al., Reference Fitzmaurice, Laird and Ware2011; Laird & Ware, Reference Laird and Ware1982). Likelihood ratio tests supported this random-effects structure (see Table S2 in Supplement).
Our first model examined the “unique” effects of life experiences and PRSs on each cognitive bias. We designated these effects as fixed and in separate models. In the life experiences model, we adjusted for the highest PRS threshold 1.0 (to adjust for possible confounding genetic effects). In each PRS model (nine, in total), we adjusted for both negative and positive life experiences (PLEs) (i.e., the environmental effects).
In our second model, we examined the interaction between life experiences and the genetic risk scores (at all 9 thresholds), while adjusting for their independent effects. In this model, we considered significant G × E (interaction) effects to be supportive of the CogBIAS hypothesis and followed them up using simple slopes for individuals at the lowest and highest polygenic thresholds (e.g., Assary et al., Reference Assary, Vincent, Keers and Pluess2018).
Age, sex, depression, and anxiety were included as covariates in all models. Where significant, time effects (see Appendix S3) were further included as covariates. To account for multiple testing, we adjusted our p values at 5%, using the Benjamini–Hochberg false discovery rate (FDR) method (Benjamini & Hochberg, Reference Benjamini and Hochberg1995). Missing values (at Wave 3) were assumed to be missing at random and handled via maximum likelihood estimation. Our analyses were conducted in STATA-13 (StataCorp, 2011).
Sensitivity analyses. To examine the robustness of our results, we performed several sensitivity checks. First, we examined whether some control variables were not confounding but instead collider. An example collider case could involve depression, which instead of exerting “causal” effects on predictors (e.g., life experiences) and outcomes (i.e., biases), it could be “caused” by them (e.g., Elwert & Winship, Reference Elwert and Winship2014). To examine potential collider cases, we re-fitted all models excluding psychopathology (see Appendix S6).
Second, although a particular strength of the CASE measure for life experiences is the option for respondents to rate the valence (i.e., positive, or negative) of life experiences, descriptive analyses revealed substantial variability in the manner by which respondents rated certain items (e.g., “I stayed away from home overnight” could either be described as a very positive or a very negative life experience). Inferential analyses further revealed that cognitive biases “explained” significant variation in some of these items, suggesting that certain life experiences could have been “contaminated” by cognitive biases (as their responding was, at least partly, a function of these biases). To check the robustness of our findings, vis-à-vis this case of reverse-causality, we repeated our significant analyses without these contaminated items (see Appendix S7).
Finally, another possibility of reverse-causality is cognitive biases giving rise to certain life experiences. Indeed, the CASE measure makes a distinction between independent events (which occur independent-of-someone’s-actions, e.g., “Someone in my family died”), dependent events (which are, at least partly, dependent on someone’s actions, e.g., “I broke up with my boyfriend/girlfriend”), and ambiguous events (which could be either of those, e.g., “I changed schools”) (see Allen & Rapee, Reference Allen and Rapee2009). Since the latter two sets of life events could, at least partly, ensue due to cognitive biases, we repeated our significant analyses by excluding “dependent,” “ambiguous,” and “contaminated” life experiences (see Appendix S8).
Results
Descriptive statistics are outlined at Table 1. The internal consistency (or reliability) of our measures ranged from acceptable (ω < 75) to high (ω > 80). Upon accounting for attrition, our final sample comprised N = 337 individuals at Waves 1 and 2, and N = 311 at Wave 3, all of whom were White Europeans (to accommodate our genetic analyses). Missing data at Wave 3 were handled via maximum-likelihood. Included participants did not differ significantly from excluded ones on any measures (see Table S1).
Effects of life experiences on cognitive biases
Positive Life Experiences (PLEs) were associated with lower Memory and Social Interpretation Biases , at FDR-adjusted p values. Crucially, these effects were driven by the positive components of these biases, that is, the Positive Recall and Positive Social Interpretation, respectively, which were both positively associated with PLEs (see Table 2). These effects were robust to a sensitivity check, which adjusted for the most significant PRS threshold (i.e., PRS 0.01) (see Appendix S4). There was only one effect from negative life experiences (on the Positive Social Interpretation); however, this effect did not survive our sensitivity analyses (please see sensitivity analyses section). (Additional effects from negative life events on cognitive biases were also detected in supplementary models that did not adjust for psychopathology (Table S7); however, these effects did not remain significant upon adjusting for psychopathology (see Appendix S10 for non-significant findings)).
Table 2. Effects of positive/negative life experiences on cognitive biases
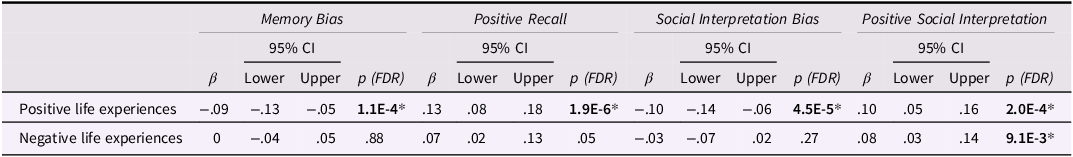
Note. β, standardized regression coefficient in SD; 95% CI, confidence intervals; p, FDR-adjusted p value for regression coefficient.
Each cognitive bias (outcome) was regressed, in turn, on negative and positive life experiences in a random intercepts model, while adjusting for age, gender, time, and depression and anxiety scores.
*p < .05 (two-sided) after Benjamini–Hochberg FDR adjustment.
Effects of polygenic scores on cognitive biases
Our polygenic score for depression was not significantly associated with any cognitive biases (at none of its nine PRS thresholds), in neither our environment-adjusted nor environment-unadjusted models (see Appendix S5). In our psychopathology-unadjusted models, we found that some polygenic thresholds had positive effects on Negative Recall, and negative effects on Positive Recall and Positive Social Interpretation (see Table S8). However, as with the effects of negative life experiences, these polygenic effects were not significant after adjusting for psychopathology (see Appendix S10 for non-significant findings).
Interactive polygenic-by-experience effects on cognitive biases
Cross-over interaction effects between polygenic risk and life experiences (G × E) were found between PLEs and various polygenic thresholds for Social Interpretation Bias and Positive Social and Non-Social Interpretation (Table 3). In particular, PLEs interacted with PRSs at eight thresholds (all except the lowest, 0.001) to predict a lower Social Interpretation Bias and a greater Positive Social Interpretation; and at four thresholds (0.01, 0.05, 0.1, and 0.2) to predict a greater Positive Non-Social Interpretation. Crucially, no significant G × E interaction effects were found between polygenic scores and negative life events (although some effects existed in supplementary models that did not adjust for psychopathology) (see Tables S11 and S12).
Table 3. G × E interaction effects (across all polygenic thresholds) on cognitive biases
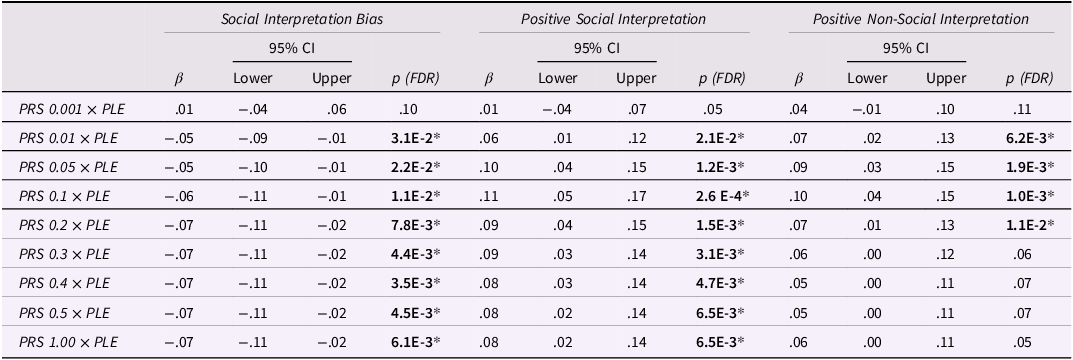
Note. PRS, polygenic risk score; PLE, positive life experiences; β, standardized regression coefficient in SD; 95% CI, confidence intervals; p, p value for regression coefficient.
Each cognitive bias (outcome) was regressed on the denoted (fixed) G × E interaction effect in a random intercepts model, while adjusting for age, gender, random time, depression, and anxiety scores.
*p < .05 (two-sided) after Benjamini–Hochberg FDR adjustment.
To understand the nature of significant interactions, we calculated simple slopes for those individuals who scored at the high- and low-PRS quartiles (Figure 1). Results from these analyses suggested that the above interaction effects were only significant at the high-PRS quartile (Table 4). Notably, this pattern was most robust for the Social Interpretation Bias and its positive component (both significant at eight PRS thresholds), and less robust for the Positive Non-Social Interpretation (which was significant at four PRS thresholds and did not survive our sensitivity tests) (see next section).

Figure 1. Simple slopes for those individuals at the low and high quartiles of polygenic risk for depression (at 0.1 threshold, the most robust of all PRS thresholds in the G × E analyses). These results suggest that the G × E interaction effects were significant only for those individuals at the high-PRS quartile, thereby supporting the notion of (vantage) sensitivity.
Table 4. Simple slope analysis denoting the significant G × E interaction effects, conditional on the high polygenic risk/sensitivity quartile

Note. PRSs, polygenic risk scores (at high quartile); PLE, positive life experiences; β, standardized regression coefficient in SD; 95% CI, confidence intervals; p, p value for regression coefficient.
Simple slopes of the significant G × E interaction effects, conditional on the high-PRS quartile, in a random intercepts model while adjusting for age, gender, random time, depression and anxiety scores. No significant G × E interaction effects were detected at the lowest PRS quartile, hence their exclusion from the table.
*p < .05 (two-sided) after Benjamini–Hochberg FDR p value adjustment.
Sensitivity analyses
To ensure robustness of the above results, we ran three sensitivity analyses. First, to explore the possibility of false positive patterns due to “collider” effects, we re-fitted all models without adjusting for psychopathology. Apart from some patterns becoming significant (due to the exclusion of psychopathology), these analyses revealed that one previous effect, from negative life experiences to the Positive Social Interpretation, was no longer significant. This suggests that our psychopathology variables may have induced a spurious relation between negative life experiences and this positive bias, by not being a true confounder, but rather a collider variable (see Appendix S6 for more information).
Second, we distinguished “contaminated” and “uncontaminated” life experiences and repeated our main analyses without the former. Contaminated items were those that were significantly predicted by at least one cognitive bias. Logistic regression analyses revealed nine such items (out of 38) (see Table S14). Repeating our previously significant analyses without these 9 “contaminated” life experiences revealed two “null” results. Namely, the above “collider” pattern (Table S16) and the positive G × E effect toward the Positive Non-Social Interpretation (see Tables S17 and S18).
Finally, we repeated our analyses, excluding the “contaminated,” “dependent,” and “ambiguous” life events. Dependent life events in the CASE measure have been defined as those events that are, to some extent, influenced by an individual’s behavior, while the ambiguous ones are “indeterminate” (Allen and Rapee, Reference Allen and Rapee2009). By excluding the “contaminated” (9 events), “dependent” (14 events), and “ambiguous” (4 events) items, we reduced the original CASE measure to 18 items (see Table S19). Despite reduced power, most effects remained significant, except for the two abovementioned (see Tables S21−S23).
Discussion
Across adolescence, the number of positive, but not negative, life experiences was associated with the development of cognitive biases. A polygenic risk for depression was not significantly associated with any cognitive biases after adjusting for psychopathology, but its G × E interaction with positive life experiences was related to a social interpretation bias. Simple slopes further revealed that G × E interaction effects were driven by genetic “risk,” as they were only significant for those individuals who scored at the highest polygenic quartile.
To our knowledge, this is the first study to demonstrate the effects of positive life experiences on cognitive biases. Positive events were shown to enhance positive recall and positive social interpretation. Recent research has validated the protective qualities of these biases. For instance, a 1 year, longitudinal study showed that a “bias” to recall positive memories predicted lower levels of cortisol and negative cognitions during stressful periods in adolescence (Askelund et al., Reference Askelund, Schweizer, Goodyer and van Harmelen2019). Similarly, a randomized control trial of a brief psychosocial intervention suggested that emphasizing the value of positive social experiences can lead to a clinically significant reduction of depressive symptoms in adolescents (see Goodyer et al., Reference Goodyer, Reynolds, Barrett, Byford, Dubicka, Hill, Holland, Kelvin, Midgley, Roberts, Senior, Target, Widmer, Wilkinson and Fonagy2017). Our results extend these findings by supporting the CogBIAS notion that positive life events could promote these “wellbeing precursors” (see Fox & Beevers, Reference Fox and Beevers2016). Crucially, our results further implicated the absence of positive life events to the presence of negative cognition. This has important implications for psychopathology research in general, and the CogBIAS model, in particular, as it suggests that the absence of positive life experiences (not merely the presence of negative ones) could be a significant contributor to psychopathology during (and perhaps beyond) adolescence.
Against our preregistered hypotheses, negative life experiences were not associated with any cognitive biases. These patterns run counter not only to the CogBIAS hypothesis, but also a wealth of empirical evidence implicating negative environmental effects in the development of cognitive biases (e.g., Pine et al., Reference Pine, Mogg, Bradley, Montgomery, Monk, McClure, Guyer, Ernst, Charney and Kaufman2005; Zavos et al., Reference Zavos, Gregory and Eley2012). We consider two possibilities for this inconsistency. First, in our unadjusted models, negative life experiences were related to several cognitive outcomes; however, these effects were not significant upon adjusting for psychopathology. Although conclusions regarding temporal precedence cannot be drawn, a potential interpretation could be that current negative circumstances do not influence the development of cognitive biases, over and above the effects of (preexisting) psychopathology. This interpretation is also supported by a supplementary structural equation model, which suggested that psychopathology partly mediates the effects of negative life events to cognitive biases (see Appendix S4).
A second possibility for the nonsignificant effects of negative life experiences may be that their operationalization was not severe “enough” for the proliferation of cognitive biases. Indeed, some of the negative life experiences in the CASE measure appear to reflect more “common,” rather than “severe,” adversity, for example, “I broke up with my boyfriend / girlfriend.” If replicated, this “null” pattern could provide evidence for prominent accounts on resilience or antifragility which designate moderate, but not severe, adversity as a precursor of resilience, not psychopathology (e.g., see Rutter, Reference Rutter2012; Taleb, Reference Taleb2014).
Relatedly, we found no main polygenic effects onto our cognitive biases, in neither our adjusted nor unadjusted models. Although some diathesis-stress patterns (i.e., negative G × E interactions) were found in supplementary unadjusted models (see Appendix S6), these effects were also no longer significant upon adjusting for psychopathology. Previous studies have showcased that polygenic effects attenuate upon adjusting for (general or PRS-specific) psychopathology (Brikell et al., Reference Brikell, Larsson, Lu, Pettersson, Chen, Kuja-Halkola, Karlsson, Lahey, Lichtenstein and Martin2020; Waszczuk et al., Reference Waszczuk, Miao, Docherty, Shabalin, Jonas, Michelini and Kotov2021). Moreover, while some polygenic scores have achieved endophenotypic predictions (e.g., attention-deficit or externalizing PRS), others appear to be more disorder-specific (Docherty et al., Reference Docherty, Moscati, Dick, Savage, Salvatore, Cooke, Aliev, Moore, Edwards, Riley, Adkins, Peterson, Webb, Bacanu and Kendler2018; Krapohl et al., Reference Krapohl, Euesden, Zabaneh, Pingault, Rimfeld, von Stumm, Dale, Breen, O’Reilly and Plomin2016; Luciano et al., Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali, Harris, Marioni, Liewald, Fawns-Ritchie, Adams, Howard, Lewis, Gale, McIntosh and Deary2018; Waszczuk et al., Reference Waszczuk, Miao, Docherty, Shabalin, Jonas, Michelini and Kotov2021). Finally, although it has sometimes been argued that direct genetic effects are necessary for interactive genetic effects (G × E), research later established that the two are relatively independent (Caspi et al., Reference Caspi, Hariri, Holmes, Uher and Moffitt2010; Dick, Reference Dick2011); indeed, interactive effects are often amplified, and thus more easily detected, than main genetic effects (see Manuck and McCaffery, Reference Manuck and McCaffery2014; Moffitt et al., Reference Moffitt, Caspi and Rutter2006). Our “null” PRS effects could reflect any of these patterns. Future studies could therefore examine similar polygenic effects using other polygenic scores (e.g., anxiety; Purves et al., Reference Purves, Coleman, Meier, Rayner, Davis, Cheesman, Bækvad-Hansen, Børglum, Wan Cho, Jürgen Deckert, Gaspar, Bybjerg-Grauholm, Hettema, Hotopf, Hougaard, Hübel, Kan, McIntosh, Mors and Eley2020); at different developmental periods (for instance, during early development, such as childhood, when the confounding effects of psychopathology are less pronounced) (Riglin et al., Reference Riglin, Thapar, Leppert, Martin, Richards, Anney, Davey Smith, Tilling, Stergiakouli, Lahey, O’Donovan, Collishaw and Thapar2020); and with larger and more diverse samples (to ensure high statistical power) (Dudbridge, Reference Dudbridge2013).
Finally, these limitations notwithstanding, a significant interaction was found between a high polygenic risk for depression and a greater number of positive life events on the social interpretation bias and its positive component. Notably, these genetic effects were protective; driven by the positive social component of the interpretation bias; and strongest for those who scored highest on polygenic risk, in line with the CogBIAS notion of (vantage) sensitivity. To our knowledge, this is the first time a psychiatric polygenic score has been associated with positive psychological outcomes, supporting the vantage sensitivity hypothesis (i.e., positive genetic effects). Although somewhat paradoxical, this pattern can be interpreted in at least two ways. First, our adjustment of internalizing psychopathology could have rendered our polygenic predictions predominantly positive by adjusting for their negative components (i.e., the depressive phenotype accounting for its genotypic counterpart, leaving only positive genetic variation). This interpretation was, in fact, supported by our unadjusted analyses, which indicated that upon excluding psychopathology from our models, the positive polygenic effects were minimized (please see Appendix S6).
Notably, though, these effects were not minimized to insignificance, suggesting that the adjustment of some negative polygenic effects was not the sole reason for their positive effect. In light of this, an alternative interpretation of these positive genetic effects could concern the depressive genotype, itself; in particular, that it might confer sensitivity to prosperity. Albeit somewhat speculative, this interpretation is in keeping with a growing corpus of data suggesting that people with a propensity for, or diagnosis of, major depression can exhibit outcomes in a “for better or for worse” manner: they are more vulnerable in response to adversity, yet also more resilient in response to prosperity, compared to their nondepressed counterparts (see Belsky et al., Reference Belsky, Bakermans-Kranenburg and van IJzendoorn2007; Belsky & Pluess, Reference Belsky and Pluess2009; Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn2011 for reviews). That being noted, we must stress nevertheless that previous polygenic studies on the matter (i.e., Chen et al., Reference Chen, Chen, Moyzis, Stern, He, Li, Li, Zhu and Dong2011; Keers et al., Reference Keers, Coleman, Lester, Roberts, Breen, Thastum, Bögels, Schneider, Heiervang, Meiser-Stedman, Nauta, Creswell, Thirlwall, Rapee, Hudson, Lewis, Plomin and Eley2016; Keers & Pluess, Reference Keers and Pluess2017) have demonstrated vantage genetic effects using polygenic scores of “environmental sensitivity,” not psychiatric illness. This discrepancy should be noted when interpreting our own vantage effect, which must be casted as “suggestive,” rather than “conclusive,” and, of course, replicated in order to provide strong support for the vantage sensitivity hypothesis. Nevertheless, it is worth noting that this effect survived our sensitivity tests, suggesting that it is unlikely to be a function of collider and reverse-causality biases.
Strengths, limitations, and future directions
The main strength of our study lies in its comprehensive assessment of both positive and negative gene-by-environment effects. Our results extend a literature that has so far been constrained to the negative spectrum of psychological development and encourage the application of a (vantage) sensitivity approach to other phenotypes.
Regarding limitations, we acknowledge our operationalization of adversity (which might have been more “common” rather than “severe”), as well as the confounding effects of psychopathology. Future research could examine at what level of adversity negative cognitive biases proliferate and do so particularly at earlier developmental periods (e.g., childhood), when the confounding effects of psychopathology are minimized. It would similarly be beneficial to examine specific vantage, stress, and sensitivity effects, using separate polygenic sets, as well as in larger and more diverse samples.
To some extent, our study was a “proof-of-a-concept” one, aimed to explore broad patterns of genetic sensitivity (using a single PRS for depression) on hitherto unexamined cognitive phenotypes. Nonetheless, accruing research has begun amalgamating the candidate gene and polygenic approaches to assemble more homogenous polygenic sets (e.g., Pluess, Reference Pluess2015). Future research could utilize these genetic sets to scrutinize specific vantage or stress effects. Such polygenic sets could also be preferably derived from adolescent-specific GWAS, although we do recognize that sample sizes from such GWAS tend to be smaller (and so, less well powered) than adult GWAS. Our study sample was also relatively small and restricted to individuals with European ancestry. With the advent of GWAS in diverse populations, replication of our polygenic effects in non-European cohorts will be of vital importance (Roberts et al., Reference Roberts, Khoury and Mensah2019).
Conclusion
To conclude, our results partly support but also extend the CogBIAS model. First, the effects of life experiences on cognitive biases (and psychopathology, more broadly) might be more nuanced than previously thought. (For instance, the absence of PLEs may lead to negative cognition, and more severe adversity may be required for the proliferation of negative biases.) A second implication of our results concerns the confounding effects of psychopathology in polygenic predictions, and the use of larger samples, varied polygenic scores, and examinations of different developmental periods as remedies. Our final, and perhaps most important, implication includes the interaction of such psychiatric polygenic scores with positive, not negative, life experiences in the prediction of positive psychological outcomes, across adolescence. If replicated, this positive G × E effect could support the notion of vantage sensitivity, painting a more holistic but also optimistic picture of psychological development.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423001645.
Acknowledgements
The authors are grateful to the European Research Council (ERC) who funded the CogBIAS research project by means of an Advanced Investigator Award to Elaine Fox (grant agreement number: 324176, under the European Union’s Seventh Framework Programme), when she was based at the University of Oxford. We would also like to acknowledge our dear friend and colleague, Dr Rob Keers, who guided us through the complexities of combining genetic and cognitive approaches, and who sadly passed away in 2020. His influence remains strong as we continue to analyze data from the CogBIAS project.
Funding statement
None.
Competing interests
None.







