Introduction
Child maltreatment in the form of physical, emotional, and sexual abuse as well as physical and emotional neglect has an unfortunately high prevalence (Stoltenborgh, Bakermans-Kranenburg, Alink, & van IJzendoorn, Reference Stoltenborgh, Bakermans-Kranenburg, Alink and van IJzendoorn2015). Substantial evidence from epidemiological and clinical studies suggests that exposure to maltreatment strongly and robustly alters emotional and physiological stress regulation (Engel & Gunnar, Reference Engel and Gunnar2020; Herzberg & Gunnar, Reference Herzberg and Gunnar2020) and increases the risk for psychiatric diseases, including depression and anxiety disorders, impaired cognitive development, and for chronic physical disease outcomes, including cardiovascular disease, obesity, diabetes, lung cancer, chronic pain, headaches, and immune-related diseases, resulting in reduced longevity (Brown et al., Reference Brown, Anda, Tiemeier, Felitti, Edwards, Croft and Giles2009; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998; Heim & Binder, Reference Heim and Binder2012; Norman et al., Reference Norman, Byambaa, De, Butchart, Scott and Vos2012; Wegman & Stetler, Reference Wegman and Stetler2009). Based on the considerations that in the majority of these diseases inflammation plays a dominant pathological role, inflammation has been proposed as a mechanism through which early adverse experiences may become ‘biologically embedded’ (Danese & Baldwin, Reference Danese and Baldwin2017; Danese & McEwen, Reference Danese and McEwen2012; Miller, Chen, & Cole, Reference Miller, Chen and Cole2009).
A large number of retrospective studies have provided evidence for elevated concentrations of inflammation-related biomarkers in adults with a history of maltreatment (reviewed in Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli2016; Coelho, Viola, Walss-Bass, Brietzke, & Grassi-Oliveira, Reference Coelho, Viola, Walss-Bass, Brietzke and Grassi-Oliveira2014). However, most of these studies in adults have been cross-sectional in design and rely on retrospective self-reports of childhood maltreatment exposure, not allowing for causal inferences, nor do they inform about early embedding processes. A few longitudinal studies exist that report associations between childhood maltreatment and increased markers of inflammation assessed in early adulthood (Danese, Pariante, Caspi, Taylor, & Poulton, Reference Danese, Pariante, Caspi, Taylor and Poulton2007; Rasmussen et al., Reference Rasmussen, Moffitt, Eugen-Olsen, Belsky, Danese, Harrington and Caspi2019). However, these studies did not collect biological data in the immediate aftermath of exposure to maltreatment and across development. So far only very few studies have provided evidence that the association between childhood maltreatment and higher levels of inflammation can already be observed during childhood. Danese et al. (Reference Danese, Caspi, Williams, Ambler, Sugden, Mika and Arseneault2011) reported that inflammation levels in maltreated children who developed depression were already elevated by age 12 compared to controls. Furthermore, higher C-reactive protein (CRP) levels were found in 10-year old children with recent onset of maltreatment and a genotype predisposing to higher CRP levels (Cicchetti, Handley, & Rogosch, Reference Cicchetti, Handley and Rogosch2015). A prospective longitudinal study showed that exposure to adverse events before the age of 8 is associated with elevated CRP concentrations at the age of 10 and 15 years (Slopen, Kubzansky, McLaughlin, & Koenen, Reference Slopen, Kubzansky, McLaughlin and Koenen2013). To the best of our knowledge, only one study so far investigated the relationship between exposure to childhood adversity and inflammation in preschool-aged children (3–5 year old) and found higher concentrations of a pro-inflammatory cytokine (IL-1β) in relation to higher exposure to childhood adversity (Tyrka, Parade, Valentine, Eslinger, & Seifer, Reference Tyrka, Parade, Valentine, Eslinger and Seifer2015). Findings from the E-Risk study suggest that there may be sex differences in the vulnerability to develop a pro-inflammatory state after childhood maltreatment exposure (Baldwin et al., Reference Baldwin, Arseneault, Caspi, Fisher, Moffitt, Odgers and Danese2018): in females, CRP concentrations assessed at the age of 18 years were significantly associated with childhood victimization, while no significant association was observed in males. Evidence from experimental studies in animals supports the notion of sex-specific effects of early-life stress on inflammation (reviewed in Ganguly & Brenhouse, Reference Ganguly and Brenhouse2015), and findings in humans suggest that, in general, women may be more sensitive than men to the effects of stress in eliciting a pro-inflammatory response (Rampp et al., Reference Rampp, Eichelkraut, Best, Czamara, Rex-Haffner, Uhr and Menke2018; Rohleder, Schommer, Hellhammer, Engel, & Kirschbaum, Reference Rohleder, Schommer, Hellhammer, Engel and Kirschbaum2001).
Thus, given the limited number of studies on the effects of childhood maltreatment on inflammation in younger children and evidence that this association may be moderated by sex, in the present study we investigated the association between childhood maltreatment and inflammation and the role of child sex in this context in a population of 3–5 year-old children that were recruited in the immediate aftermath of maltreatment and were followed-up longitudinally every 6 months over a period of 2 years, and in a nonexposed control group. At each assessment time point, salivary concentrations of CRP were assessed. (CRP is produced in the liver in response to interleukin-6 secretion by macrophages and T cells and is therefore considered a biomarker of systemic inflammation.)
We hypothesized that (a) maltreated children show increased CRP concentrations compared to the control group; (b) the association between maltreatment and CRP is moderated by child sex; and (c) the observed differences between the maltreatment and comparison group remain stable over the course of the 2-year follow-up period.
Method
Recruitment, study sample, and design
This report is part of a larger research study entitled “Immediate Biological Embedding of Maltreatment in Children: The Berlin Longitudinal Children Study (BerlinLCS)” that was funded by Federal Ministry of Education and Research (01KR1301). The present report presents data on inflammatory markers obtained in this study.
Approval for the study was obtained from the ethics committee of Charité – Universitätsmedizin Berlin. All procedures are in accordance with the Ethical Principles for Medical Research as established by the Medical Association Declaration of Helsinki. Written informed consent was obtained from all participants after the procedures were fully explained. Children gave consent by painting or signing a form that was appropriate for the children's age range.
A total of 173 children aged 3 to 5 years and their caregivers were recruited at study entry (T0, see Table 1 for sample characteristics). The sample includes 86 children who had been exposed to maltreatment within 6 months before study entry and 87 nonmaltreated children. Maltreatment and control groups were frequency-matched for sex and age. General exclusion criteria for all children included parents under the age of 18 years, moderate or severe mental intellectual dysfunction or neurodevelopmental disorders, chronic medical illness as well as serious medical disease of the parents. The absence of fever or acute severe infection at the time the sample was taken for CRP analyses was confirmed during a medical exam.
Table 1. Sample characteristics of children in the control and maltreatment group at baseline (T0)
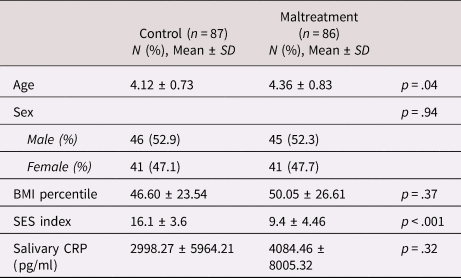
Note. CRP, C-reactive protein; SD, standard deviation; SES, socioeconomic status.
Maltreated children were recruited from a broad range of local child welfare and protection services, pediatric offices, and through advertisements and letters sent to families with children aged 3–5 years mainly identified through public census records. Nonmaltreated children were recruited from the community using advertising as well as letters directed to families with children between 3–5 years of age using census records. For assignment to the control group, children were screened to exclude exposure to any severe critical or traumatic life event.
The study employed a prospective, longitudinal design with serial assessments of the children with follow-up visits scheduled after an initial baseline assessment (T0, maltreatment group: n = 86, control group n = 87) at 6 months (T1, maltreatment group: n = 65, control group: n = 80), 12 months (T2, maltreatment group: n = 60; control group: n = 70), 18 months (T3, maltreatment group: n = 47, control group n = 64), and 24 months (T4, maltreatment group n = 35, control group n = 52).
Measures
Childhood maltreatment
The occurrence and features of maltreatment were assessed at T0. At each follow-up visit, the assessment was repeated for the interim time for safety purposes. To assess features of maltreatment, we used the Maternal Interview for the Classification of Maltreatment (Cicchetti, Toth, & Manly, Reference Cicchetti, Toth and Manly2003). The interview was administered by trained clinicians and based on caregiver reports. Responses were coded according to the Maltreatment Classification System (Barnett, Manly, & Cicchetti, Reference Barnett, Manly, Cicchetti, Cicchetti and Toth1993), which provides specific criteria for classifying and quantifying the occurrence and features of subtypes of maltreatment. The interview covers a range of subtypes, including emotional maltreatment (i.e. emotional abuse and/or emotional neglect), physical neglect (i.e. failure to provide and/or lack of supervision), physical abuse, and moral, legal and/or educational maltreatment. In our sample, the latter subtype overlapped with lack of supervision due to excessive video gaming or keeping the child busy, which we therefore assigned to the physical neglect category. For each incidence, severity is rated on a 5-point scale ranging from mild (1) to severe or life-threatening maltreatment (5). Severity cutoff scores were used to include children in the maltreatment group (emotional maltreatment ≥2, physical abuse ≥1, physical neglect ≥2). We did not specifically recruit for sexual abuse, as sexual abuse usually leads to removal of the child from the home, which we considered would be a significant intervention; however, co-incident mild forms of sexual abuse were detected in 11 of 86 maltreated children.
Salivary C-reactive protein (CRP)
Due to the noninvasiveness of sampling, salivary CRP sampling constitutes a feasible measure to study peripheral inflammation in children (Cicchetti et al., Reference Cicchetti, Handley and Rogosch2015). Several studies (Ouellet-Morin, Danese, Williams, & Arseneault, Reference Ouellet-Morin, Danese, Williams and Arseneault2011; Out, Hall, Granger, Page, & Woods, Reference Out, Hall, Granger, Page and Woods2012) report moderate to strong associations between serum and salivary CRP levels. Saliva samples were collected during each clinical visit at 11 a.m. by oral swabs specially designed for small children (Salimetrics) and were immediately stored at −80 °C. Salivary CRP was determined using a commercial ELISA kit (Salimetrics) with a sensitivity of 10 pg/ml. Intra-assay and inter-assay coefficients of variability were 6% and 13%, respectively.
Insufficient amounts of saliva for CRP analyses were collected for some children. The final sample size with valid CRP measurements included in the analyses is as follows: T0, maltreatment group: n = 86; control group n = 82; T1, maltreatment group: n = 64, control group n = 80; T2, maltreatment group: n = 59, control group: n = 70; T3, maltreatment group: n = 43, control group n = 64; T4, maltreatment group: n = 35, control group: n = 52.
Covariates and potential confounders
Socioeconomic status (SES)
SES was estimated based on a modification of the Winkler and Stolzenberg Index (Lange et al., Reference Lange, Kamtsiuris, Lange, Schaffrath Rosario, Stolzenberg and Lampert2007). This multidimensional index score represents the sum of three metric components: education and occupational qualification, occupational status, and net income. The score reflects low (score 3 to 8), middle (9–14), or high (15–21) SES of the participating family.
Body mass index (BMI)
Child's body weight and length were measured by a physician during a physical examination at each clinical visit. BMI percentiles were calculated according to German clinical standards (Rosario, Kurth, Stolzenberg, Ellert, & Neuhauser, Reference Rosario, Kurth, Stolzenberg, Ellert and Neuhauser2010).
Data analysis
t tests and chi-square tests were used to compare the maltreatment and control group regarding demographics and clinical characteristics at baseline (T0, see Table 1). We observed significant differences in age (t = 2.07, p = .04) and SES (t = −10.91, p < .001) such that the maltreatment group was slightly older and of lower SES compared to the control group. The differences in age and SES remained constant over all clinical time points. At T1–T3, the maltreatment group had a higher BMI compared to controls (T1, t = 2.02, p = .046; T2, t = 2.44, p = .016; T3; t = 2.16, p = .015). In order to account for the possible confounding influence of age, SES, and BMI, these covariates were included in all statistical models.
CRP measures were first log transformed to bring outliers closer to the mean. Linear mixed effects models were employed to determine the associations between maltreatment exposure and CRP concentration across all visits. Restricted maximum likelihood estimation was performed. These models allow inclusion of all subjects with incomplete measurements in the repeated measures variables. This method does not impute any data, but rather uses each case available to compute maximum likelihood estimates, thereby minimizing biased estimates (Snijders & Bosker, Reference Snijders and Bosker2012). The final sample included in the mixed effects model consisted of N = 173 children. The models included a random intercept term as well as an autoregressive (AR(1)) heterogeneous covariance structure in order to account for the expected within-subject correlation of CRP across time (clinical visits).
To examine group differences between the maltreatment and comparison group across all time points, group status was entered as the predictor, along with the covariates age, SES and BMI, and child sex (main effect model, Model 1).
In a second step, to test effect modification by child sex, an interaction term between group status and child sex (Group×Child sex) was entered as an additional predictor (Model 2).
Third, to test whether the observed differences in CRP concentrations remained stable or were associated with different trajectories over time, an additional interaction term with time point (Group×Child sex×Time point) was entered (Model 3).
Results
In the main effect model (Model 1), CRP concentrations did not differ between the maltreatment and control group across all clinical visits (B = −0.030, F = −0.048, p = .827). Among the other predictors in the model, only SES was significantly and negatively associated with CRP levels (B = −0.026, F = −4.644, p = .032).
Model 2 revealed that the association between maltreatment and CRP concentrations was significantly moderated by child sex (interaction term Group×Maltreatment B = .538, F = 5.648, p = .019), such that in girls, CRP concentrations were higher across all clinical visits in the maltreatment compared to the control group, whereas in boys, there was no association between maltreatment status and CRP levels (see Table 2 and Figure 1).

Figure 1. Mean (+ SE) CRP concentration in girls and boys of the maltreatment and comparison group at each clinical visit (T0-4). CRP, C-reactive protein.
Table 2. Effect estimates of the linear mixed effects model with C-reactive protein (CRP) (Ln) as the outcome and maltreatment status, sex, Maltreatment×Sex and potentially confounding covariates as predictors
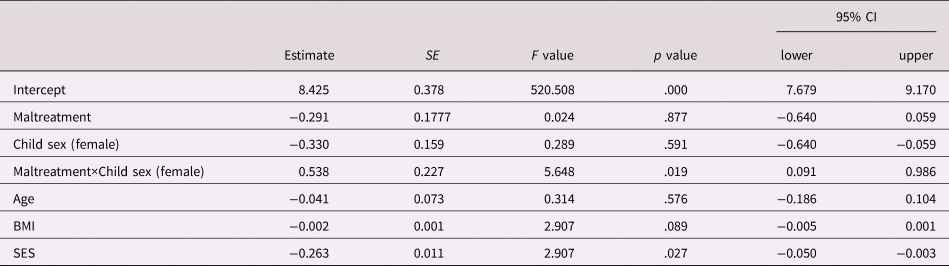
Note. Test of fixed effects, Type III; SE, standard error; CI, confidence interval; CRP, C-reactive protein; SES, socioeconomic status.
The differences in CRP concentrations between girls in the maltreatment and the comparison group were stable across all visits, as suggested by the nonsignificant coefficient of the three-way interaction Group×Sex×Time point (Model 3: B = .028, F = .316, p = .814).
Discussion
We here prospectively examined the association between childhood maltreatment and inflammation in the immediate aftermath of the exposure and over a 2-year follow-up period in very young children (3–5 years age). Our results suggest a sex-specific effect of childhood maltreatment exposure on CRP concentrations, such that after accounting for potential confounders like SES, BMI, and age, maltreated girls exhibited higher CRP concentrations at study entry and over a 2-year follow-up period compared to nonexposed girls, whereas there was no association between maltreatment and CRP in boys.
Our findings are in line with those of earlier studies that show associations between childhood adversity and a pro-inflammatory states in adults, adolescence, and children (Baumeister et al., Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli2016; Cicchetti et al., Reference Cicchetti, Handley and Rogosch2015; Coelho et al., Reference Coelho, Viola, Walss-Bass, Brietzke and Grassi-Oliveira2014; Danese et al., Reference Danese, Pariante, Caspi, Taylor and Poulton2007; Danese et al., Reference Danese, Caspi, Williams, Ambler, Sugden, Mika and Arseneault2011;Rasmussen et al., Reference Rasmussen, Moffitt, Eugen-Olsen, Belsky, Danese, Harrington and Caspi2019; Slopen et al., Reference Slopen, Kubzansky, McLaughlin and Koenen2013; Tyrka et al., Reference Tyrka, Parade, Valentine, Eslinger and Seifer2015). We here find that this effect emerges right after exposure in very young children. We replicate findings from previous studies that suggest that lower SES is associated with increased inflammation (see Milaniak & Jaffee [Reference Milaniak and Jaffee2019] for a recent meta-analysis). Our study tested for sex-specific effects of early-life stress on inflammation at this very early age, and our observation of a sex-specific effect of childhood maltreatment on CRP concentrations replicates findings from a recent study in young adults (Baldwin et al., Reference Baldwin, Arseneault, Caspi, Fisher, Moffitt, Odgers and Danese2018).
There are several potential pathways and mechanisms through which exposure to childhood maltreatment can induce a pro-inflammatory state (Danese & Lewis, Reference Danese and Lewis2017): acute stress through stimulation of the sympathetic nervous system can prompt an acute inflammatory response (Prather et al., Reference Prather, Carroll, Fury, McDade, Ross and Marsland2009; Steptoe, Hamer, & Chida, Reference Steptoe, Hamer and Chida2007) and induces the up-regulation of the transcription factor NF-κB, a key regulator of inflammation, in circulating immune cells (Bierhaus et al., Reference Bierhaus, Wolf, Andrassy, Rohleder, Humpert, Petrov and Nawroth2003). Short-term activation of the inflammatory response during sensitive periods in early development may affect brain development and later microglia and neuroendocrine reactivity. Furthermore, early-life stress may be indirectly linked to inflammation through neuroendocrine dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis. Childhood trauma is associated with hyperactivity of the HPA axis (Danese & McEwen, Reference Danese and McEwen2012; Gunnar & Quevedo, Reference Gunnar and Quevedo2007; Heim, Newport, Bonsall, Miller, & Nemeroff, Reference Heim, Newport, Bonsall, Miller and Nemeroff2001), presumably because of reduced sensitivity of the glucocorticoid receptor (GR), which mediates the HPA axis negative feedback. Owing to the increased resistance of the GR that expands to immune cells, the anti-inflammatory properties of cortisol are also reduced, resulting in higher inflammation levels particularly after acute stress exposure (Heim et al., Reference Heim, Newport, Heit, Graham, Wilcox, Bonsall and Nemeroff2000; Miller, Cohen, & Ritchey, Reference Miller, Cohen and Ritchey2002; Pace et al., Reference Pace, Mletzko, Alagbe, Musselman, Nemeroff, Miller and Heim2006). In addition, as proposed and reviewed by Danese and Lewis (Reference Danese and Lewis2017) psychological trauma may cooccur with physical trauma which can facilitate pathogen infection that in turn can induce inflammation. Furthermore, early-life stress has been associated with disruption of sleep, the composition of the microbiome, and self-harming behavior, all of which can contribute to a pro-inflammatory state.
Sex differences in the effects of maltreatment on inflammation may be explained by sex-related differences in HPA axis regulation and immune system function. The sexual differentiation of the brain induces neuroanatomical and physiological changes that impact the neuroendocrine stress response (McCarthy, Reference McCarthy2016), and a substantial amount of literature has reported sex differences in HPA axis activity and reactivity in adults and children (Hollanders, van der Voorn, Rotteveel, & Finken, Reference Hollanders, van der Voorn, Rotteveel and Finken2017; van der Voorn, Hollanders, Ket, Rotteveel, & Finken, Reference van der Voorn, Hollanders, Ket, Rotteveel and Finken2017). Stress-related immune activation is also affected by sex (Rampp et al., Reference Rampp, Eichelkraut, Best, Czamara, Rex-Haffner, Uhr and Menke2018; Rohleder et al., Reference Rohleder, Schommer, Hellhammer, Engel and Kirschbaum2001), inflammation-related genes exhibit unique expression profiles in males and females based on interactions between estrogen and glucocorticoid receptors (Deak et al., Reference Deak, Quinn, Cidlowski, Victoria, Murphy and Sheridan2015), and glucocorticoids do not work as potently as anti-inflammatory molecules in females versus males in response to a global inflammatory challenge (Duma, Collins, Chou, & Cidlowski, Reference Duma, Collins, Chou and Cidlowski2010). As speculated by Baldwin et al. (Reference Baldwin, Arseneault, Caspi, Fisher, Moffitt, Odgers and Danese2018), in their study in adolescence, sex hormones may partly contribute to the observed sex-specific effects because testosterone levels in male adolescents negatively correlate with CRP levels (Shanahan et al., Reference Shanahan, Copeland, Worthman, Erkanli, Angold and Costello2013). However, in our very young cohort it is unlikely that differences in sex hormones at the time of exposure may have directly contributed to the observed sex-specific effect. Developmentally programmed sex differences at this early age in brain development and immune function rather emerge through contributions of differential expression of X and Y chromosome genes in cells (Marrocco & McEwen, Reference Marrocco and McEwen2016). Furthermore, androgen exposure during earlier developmental windows (testosterone is elevated in male compared to female fetuses between about 8 and 24 weeks of gestation [Reyes, Boroditsky, Winter, & Faiman, Reference Reyes, Boroditsky, Winter and Faiman1974] and peaks again at 1–3 months of age [Winter, Hughes, Reyes, & Faiman, Reference Winter, Hughes, Reyes and Faiman1976]) may differentially imprint or prime the male and female brain and the immune system for effects of stress exposure during later developmental stages (Marrocco & McEwen, Reference Marrocco and McEwen2016). In addition, sexually dimorphic trajectories of brain development have been observed, with in general more rapid maturation in females, potentially rendering them more vulnerable to adverse exposures at this early age (Lenroot et al., Reference Lenroot, Gogtay, Greenstein, Wells, Wallace, Clasen and Giedd2007). The association between childhood maltreatment and inflammation in males as observed in other studies with older individuals may emerge later in life when males exposed to early-life stress engage in more unhealthy behaviors that are associated with inflammation (Danese & Baldwin, Reference Danese and Baldwin2017).
A key strength of the present study was the prospective, longitudinal study design, which enabled us to test for associations between maltreatment and inflammation right after exposure and over a 2-year period. Another strength involves the thorough assessment of maltreatment and the absence of it in the comparison group. Limitations are the relatively small sample size and the attrition rate over the 2-year follow-up, and that CRP concentrations were assessed in saliva and not in serum. However, moderate to strong associations between serum and salivary CRP levels have been reported (Ouellet-Morin et al., Reference Ouellet-Morin, Danese, Williams and Arseneault2011; Out et al., Reference Out, Hall, Granger, Page and Woods2012), and CRP levels and other inflammatory makers have been assessed in saliva in several other studies investigating the relationship between childhood maltreatment and inflammation (Cicchetti et al., Reference Cicchetti, Handley and Rogosch2015; Tyrka et al., Reference Tyrka, Parade, Valentine, Eslinger and Seifer2015). How these CRP trajectories in children relate to alterations in other biological system as well as clinical and developmental trajectories over time will be the aim of future, forthcoming analyses in this cohort.
Our finding of increased inflammation in young girls exposed to maltreatment has important clinical implications, because it may predispose them for a range of diseases in later life in which inflammation plays a dominant pathological role, including cardiovascular disease, cardiovascular disease, cancer, diabetes mellitus, autoimmune and neurodegenerative disorders, and psychopathology including depression (Furman et al., Reference Furman, Campisi, Verdin, Carrera-Bastos, Targ, Franceschi and Slavich2019). Moreover, the observed sex-specific association may have consequences for treatment success of depression, since depressed patients with elevated inflammation are less likely to respond to conventional antidepressants (Cattaneo et al., Reference Cattaneo, Gennarelli, Uher, Breen, Farmer, Aitchison and Pariante2013; Chamberlain et al., Reference Chamberlain, Cavanagh, de Boer, Mondelli, Jones, Drevets and Bullmore2018). We argued previously that novel intervention strategies that are mechanism driven and sensitive to developmental timing should be developed, which we can offer to victims of early adversity in order to efficiently prevent or reverse adverse health outcomes (Heim, Entringer, & Buss, Reference Heim, Entringer and Buss2019). Based on the findings of the current study, girls exposed to maltreatment might benefit from early interventions targeting inflammatory pathways, for example through anti-inflammatory agents (Rook, Raison, & Lowry, Reference Rook, Raison and Lowry2012) and interventions such as exercise (Gleeson et al., Reference Gleeson, Bishop, Stensel, Lindley, Mastana and Nimmo2011), healthy diet (Minihane et al., Reference Minihane, Vinoy, Russell, Baka, Roche, Tuohy and Calder2015), and cognitive- and meditation-based therapies (Creswell et al., Reference Creswell, Irwin, Burklund, Lieberman, Arevalo, Ma and Cole2012) to prevent or treat pathological outcomes following maltreatment exposure in early childhood.
To conclude, our findings contribute to the existing literature on the association between childhood maltreatment and inflammation, and suggest that the effect may already emerge right after exposure at a very young age in girls and manifest over time. Our study provides important evidence for the development of personalized, early intervention strategies targeting the early-life period in order to mitigate the adverse consequences of childhood maltreatment and to program lifelong health in children.
Funding Statement
This work was funded by BMBF grant 01K13101-A (to CH) and 01K13101-B (to EB).
Conflict of Interest
None






