Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
ATHERTON, MICHAEL P.
1993.
Granite magmatism.
Journal of the Geological Society,
Vol. 150,
Issue. 6,
p.
1009.
Johannes, W.
Koepke, J.
and
Behrens, H.
1994.
Feldspars and their Reactions.
p.
161.
Atherton, Michael P.
1995.
Granite magmatism.
Geological Society, London, Memoirs,
Vol. 16,
Issue. 1,
p.
221.
Carrington, D. P.
and
Harley, S. L.
1995.
Partial melting and phase relations in high-grade metapelites: an experimental petrogenetic grid in the KFMASH system.
Contributions to Mineralogy and Petrology,
Vol. 120,
Issue. 3-4,
p.
270.
Preston, R. J.
Dempster, T. J.
Bell, B. R.
and
Rogers, G.
1999.
The Petrology of Mullite-bearing Peraluminous Xenoliths: Implications for Contamination Processes in Basaltic Magmas.
Journal of Petrology,
Vol. 40,
Issue. 4,
p.
549.
White, Allan J. R.
and
Chappell, Bruce W.
2004.
Petrographic Discrimination of Low‐ and High‐Temperature I‐type Granites.
Resource Geology,
Vol. 54,
Issue. 3,
p.
215.
HOLNESS, M. B.
DANE, K.
SIDES, R.
RICHARDSON, C.
and
CADDICK, M.
2005.
Melting and melt segregation in the aureole of the Glenmore Plug, Ardnamurchan.
Journal of Metamorphic Geology,
Vol. 23,
Issue. 1,
p.
29.
Acosta-Vigil, Antonio
London, David
and
Morgan, George B.
2006.
Experiments on the kinetics of partial melting of a leucogranite at 200 MPa H2O and 690–800°C: compositional variability of melts during the onset of H2O-saturated crustal anatexis.
Contributions to Mineralogy and Petrology,
Vol. 151,
Issue. 5,
p.
539.
Kvassnes, Astri J. S.
and
Grove, Timothy L.
2008.
How partial melts of mafic lower crust affect ascending magmas at oceanic ridges.
Contributions to Mineralogy and Petrology,
Vol. 156,
Issue. 1,
p.
49.
Årebäck, H.
Andersson, U. B.
and
Petersson, J.
2008.
Petrological evidence for crustal melting, unmixing, and undercooling in an alkali-calcic, high-level intrusion: the late Sveconorwegian Vinga intrusion, SW Sweden.
Mineralogy and Petrology,
Vol. 93,
Issue. 1-2,
p.
1.
Acosta-Vigil, A.
Buick, I.
Hermann, J.
Cesare, B.
Rubatto, D.
London, D.
and
Morgan, G. B.
2010.
Mechanisms of Crustal Anatexis: a Geochemical Study of Partially Melted Metapelitic Enclaves and Host Dacite, SE Spain.
Journal of Petrology,
Vol. 51,
Issue. 4,
p.
785.
Koepke, J.
France, L.
Müller, T.
Faure, F.
Goetze, N.
Dziony, W.
and
Ildefonse, B.
2011.
Gabbros from IODP Site 1256, equatorial Pacific: Insight into axial magma chamber processes at fast spreading ocean ridges.
Geochemistry, Geophysics, Geosystems,
Vol. 12,
Issue. 9,
p.
n/a.
Acosta-Vigil, Antonio
Buick, Ian
Cesare, Bernardo
London, David
and
Morgan, George B.
2012.
The Extent of Equilibration between Melt and Residuum during Regional Anatexis and its Implications for Differentiation of the Continental Crust: a Study of Partially Melted Metapelitic Enclaves.
Journal of Petrology,
Vol. 53,
Issue. 7,
p.
1319.
Barboni, Mélanie
and
Bussy, François
2013.
Petrogenesis of magmatic albite granites associated to cogenetic A-type granites: Na-rich residual melt extraction from a partially crystallized A-type granite mush.
Lithos,
Vol. 177,
Issue. ,
p.
328.
Cesare, B.
Acosta-Vigil, A.
Bartoli, O.
and
Ferrero, S.
2015.
What can we learn from melt inclusions in migmatites and granulites?.
Lithos,
Vol. 239,
Issue. ,
p.
186.
El Dabe, Mohamed M.
2015.
A geochemical tectonomagmatic classification of the A-type granitoids based on their magma types and tectonic regimes.
Arabian Journal of Geosciences,
Vol. 8,
Issue. 1,
p.
187.
Lan, Ting-Guang
Fan, Hong-Rui
Yang, Kui-Feng
Cai, Ya-Chun
Wen, Bo-Jie
and
Zhang, Wen
2015.
Geochronology, mineralogy and geochemistry of alkali-feldspar granite and albite granite association from the Changyi area of Jiao-Liao-Ji Belt: Implications for Paleoproterozoic rifting of eastern North China Craton.
Precambrian Research,
Vol. 266,
Issue. ,
p.
86.
Acosta-Vigil, Antonio
London, David
Morgan, George B.
Cesare, Bernardo
Buick, Ian
Hermann, Jörg
and
Bartoli, Omar
2017.
Primary crustal melt compositions: Insights into the controls, mechanisms and timing of generation from kinetics experiments and melt inclusions.
Lithos,
Vol. 286-287,
Issue. ,
p.
454.
Masotta, M.
Laumonier, M.
and
McCammon, C.
2018.
Transport of melt and volatiles in magmas inferred from kinetic experiments on the partial melting of granitic rocks.
Lithos,
Vol. 318-319,
Issue. ,
p.
434.
Tavares Brasileiro, Camila
de Almeida Filho, Humberto Dias
Santana, Geovana Lira
Lot, Ana Virgínia
Conte, Sonia
Zanelli, Chiara
Dondi, Michele
and
Ortega Boschi, Anselmo
2022.
Sericite instead of feldspar in porcelain stoneware: Effect on sintering and phase evolution.
International Journal of Applied Ceramic Technology,
Vol. 19,
Issue. 1,
p.
612.
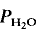 = 5 kbar, T = 1000°C) is controlled by the diffusion of water into the plagioclase structure. Melting is especially fast parallel to the a-axis. The experimental products show separation of melts and crystals.
= 5 kbar, T = 1000°C) is controlled by the diffusion of water into the plagioclase structure. Melting is especially fast parallel to the a-axis. The experimental products show separation of melts and crystals.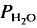 = 2 kbar) but not a lower temperatures. The kinetics of the reaction is enhanced by deficiency or excess of alumina in the aluminosilicate melt surrounding the plagioclase crystals. The fractionation of Ab and An between melt and plagioclase crystals is more pronounced in the presence of quartz than in the Ab-An-H2O system. The ratio An/An + Ab is approximately 0·35 in the melt and 0·85 in the coexisting plagioclase T = 880°C).
= 2 kbar) but not a lower temperatures. The kinetics of the reaction is enhanced by deficiency or excess of alumina in the aluminosilicate melt surrounding the plagioclase crystals. The fractionation of Ab and An between melt and plagioclase crystals is more pronounced in the presence of quartz than in the Ab-An-H2O system. The ratio An/An + Ab is approximately 0·35 in the melt and 0·85 in the coexisting plagioclase T = 880°C).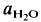 of approximately 0·5. It is assumed that near equilibrium compositions of melt and coexisting residual plagioclase could be obtained in long duration runs (run time = 60 days). The distribution of Ab and An between melt and minerals is similar to that observed in the tonalite system. The partial melt coexisting with an An-rich plagioclase and Or-rich K-feldspar is relatively poor in An.
of approximately 0·5. It is assumed that near equilibrium compositions of melt and coexisting residual plagioclase could be obtained in long duration runs (run time = 60 days). The distribution of Ab and An between melt and minerals is similar to that observed in the tonalite system. The partial melt coexisting with an An-rich plagioclase and Or-rich K-feldspar is relatively poor in An.