Introduction
In terms of infectious causes, over 100 pathogens have been identified in the aetiology of meningoencephalitis. There are a few national reports defining the epidemiology of encephalitis and there is considerable variation in geographical distribution of infectious causes [Reference Marra, Scheld, Marra, Whiteley and Scheld1]. An incidence of 4.3 cases of encephalitis/100 000 population has been reported in the UK [Reference Granerod and Crowcroft2, Reference Granerod3]. This is likely to be an underestimate, with recognised diagnostic and treatment delays contributing to substandard outcomes [Reference Kelly4–Reference Michael6]. There are a number of causes of encephalitis, including infectious, post-infectious, autoimmune, inflammatory and neoplastic, however 30–60% of cases remain undiagnosed [Reference Granerod and Crowcroft2, Reference Solomon7, Reference Glaser8]. The infectious agents responsible are predominantly viruses and bacteria; with herpes simplex encephalitis (HSE) being the most frequently reported infectious aetiology. Amongst the undiagnosed cases, the contribution of autoimmune encephalitis is increasingly acknowledged with improved clinical awareness and access to specialised diagnostic services [Reference Antoine9, Reference Granerod10]. Further, infectious aetiologies are still being identified and causative associations being better understood, with access to novel mechanisms for pathogen discovery such as next generation sequencing [Reference Calistri and Palu11].
Current UK guidelines focus on earlier initiation of aciclovir treatment, within 6 h, carrying out a lumbar puncture (LP) within 12 h and magnetic resonance imaging (MRI) of the brain within 24–48 h [Reference Solomon7]. Evidence for the role of aciclovir in improving outcomes in HSE is well-established, however, the limitations in effective treatment for other conditions are striking. Despite evidence based treatment and published guidelines, improving outcomes in encephalitis is an ongoing challenge [Reference Backman12].
We aimed to perform a retrospective evaluation of adult patients with encephalitis in a single tertiary care centre, to analyse the aetiology and outcomes, how these align with current guidelines and identify areas for which clinical management might be improved.
Methods
A retrospective evaluation of adults with encephalitis or meningoencephalitis admitted to Kings College Hospital from June 27 2013 to February 28 2014. The study included consecutive patients for whom a cerebrospinal fluid (CSF) sample had been collected and received in the virology department during the time period, for which there were available electronic records. All CSF samples underwent testing for HSV types 1 and 2 DNA, VZV DNA, CMV DNA, EBV DNA and enterovirus RNA using in-house multiplex real-time polymerase chain reaction (PCR) assays [Reference Granerod3]. A further panel of tests was added if the patients were immunocompromised or had specific imaging that suggested HHV-6 DNA, HHV-7 DNA and JCV DNA testing was required using in-house multiplex real-time PCR assays [Reference Granerod3] and HIV-1 RNA using the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 (TaqMan 2.0). Finally, if there was an indicative travel history, appropriate testing (e.g. for West Nile or Japanese encephalitis) were carried out at, St Louis Encephalitis or tick-borne encephalitis were part of the differential diagnosis, samples were sent to the Public Health England Porton Down reference laboratory. All CSF samples were also tested in bacteriology and CSF gram staining, culture and antimicrobial susceptibility testing, together with latex agglutination testing, and meningococcal and pneumococcal PCR were also performed when indicated.
The case definition for encephalitis was of altered consciousness, (lethargy, irritability, change in personality or behaviour), as well as two of the following: fever, seizures or focal neurological signs, CSF pleocytosis, specific findings on electroencephalography (EEG) or neuroimaging [Reference Venkatesan13]. The Glasgow Outcome Score was assigned from clinical documentation, if recorded, or by the study investigators using electronic records. Data collection and analysis was performed using Microsoft Excel 2013. Ethical approval was discussed with the research ethics facilitator and as this study was observational, retrospective and anonymised, approval was not needed.
Results
Thirty-six patients were identified with encephalitis/meningoencephalitis over the time period, with a median age of 55 (IQR 44–65) years; and 24 (64%) were male. Ethnicity was 27 (75%) White-British; 7 (19%) Black or Black British-African; 1 (3%) Black or Black British other and 1 (3%) Asian or Asian British Indian. Clinical presentation is demonstrated in Figure 1. The median Glasgow Coma Score was 14 (IQR 10–14). Seven (19%) reported recent infections and seven (19%) reported recent travel abroad. Twelve (33%) had underlying immunosuppression; seven (19%) had HIV and two (6%) had recently received chemotherapy. Data on timing and results of investigations is illustrated in Table 1. Fifteen (42%) patients had an abnormal white cell count (WCC) and 23 (64%) had an abnormal C-reactive protein (CRP).

Fig. 1. Clinical presentation.
Table 1. Investigationsa
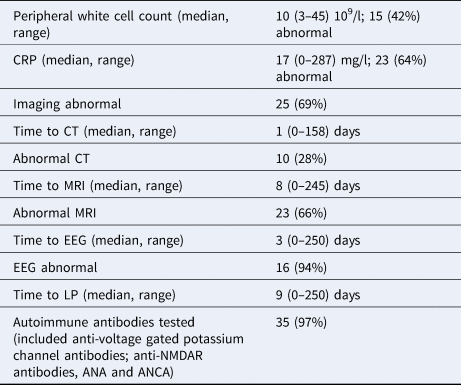
a Calculated from time of review by a medical practitioner in hospital to time of investigation.
An aetiology was identified in 25 (69%) patients. These included 16 (44%) infectious aetiologies of which 7 (28%) were viral, 7 (28%) bacterial and 2 (8%) parasitic. There was a range of pathogens identified including Neisseria meningitidis, Streptococcus pneumonia, Listeria monocytogenes, Mycobacterium tuberculosis, Treponema pallidum, pyogenic infected shunt, herpes simplex virus-2, human immunodeficiency virus, enterovirus, cerebral malaria and toxoplasmosis. There were also 7 (19%) autoimmune (anti-voltage-gated potassium channel and anti-N-methyl-d-aspartate (anti-NMDAR) antibodies), one (3%) neoplastic, acute disseminated encephalomyelitis (ADEM), central nervous system vasculitis and glioblastoma multiforme. The aetiologies of patients with underlying immunocompromise were largely infectious (8/11, 73%), however it is notable that one of these patients had confirmed anti-NMDA receptor antibody encephalitis. The remaining two with underlying immunocompromise remained undiagnosed. Aetiologies have been stratified in Table 2 as per the system published by Granerod et al., in 2010 [Reference Granerod3].
Table 2. Aetiologies presented in categories

Aciclovir was given to 19 (53%) patients on admission. The median duration of aciclovir treatment was 6 (IQR 3–9) days with a range of 0–21. Patients receiving a more prolonged course of 14–21 days fell into the category of either undiagnosed or autoimmune encephalitis. The duration of admission was a median of 32 (IQR 13–59) days. Patient outcome is shown in Table 3.
Table 3. Patient outcome at discharge evaluated by the Glasgow Outcome Score

Discussion
The high morbidity and mortality in encephalitis is well-recognised, with challenges in making an impact on outcomes over the last few decades [Reference Solomon7, Reference Backman12].
The demographics derived from these data show important differences compared to other reports from the UK. The median age was much higher at 55 as compared to 30 years, and a relatively high proportion of ethnic minorities, 25%, and immunocompromised patients at 33% as compared to 15% [Reference Granerod10]. This is likely to reflect local demographics as well as the setting being a large tertiary referral hospital with specialist neurological, neurosurgical and neuropsychiatric departments.
All patients met the case definition and nearly two thirds presented with headache and fever, and one third with seizures. Fever was seen in patients with both infectious and autoimmune encephalitis. It is important to recognize the latter as effective treatment is available.
In the emergency department, a computed topography (CT) brain scan was carried out within a median of 7 (IQR 1–14) h. However, only 10 (28%) had an abnormal CT and it is recognised that CT is not as sensitive as MRI in identifying brain abnormalities in patients with encephalitis [Reference Maschke14]. An MRI, however, was carried out within the 24–48 h time period recommended in current UK guidelines at a median of 14 (IQR 5–23) h. Median time to LP was 12 h, range 1–250, and this is 6 h longer than the recommendation. This is consistent with other reports from the UK of delays in LP, frequently due to the misunderstanding that all patients require brain imaging [Reference Bell15, Reference Brouwer and van de Beek16]. However, there are also complex issues in place surrounding workflow of patients in the emergency department, and these are not solved easily. Undoubtedly further work is needed to improve this locally.
A diagnosis was made in 25 (69%) patients, similar to other recent UK studies, and significantly higher than other studies worldwide [Reference Granerod10]. This may be due to the expertise and resources available, including the fact that almost all had autoantibody testing. In total 8/11, 73% of the cases remaining undiagnosed had autoantibody testing. However, emerging novel techniques for pathogen discovery such as 16S PCR or next-generation sequencing were not available at that time, and are yet to be validated for the routine testing of patients with encephalitis [Reference Stahl17]. Equally, there is ongoing work on point-of-care diagnostic testing that may reduce the time to diagnosis, and implementation of appropriate management [Reference McGill18].
The outcome differed depending on the aetiology: patients with infectious causes had worse outcomes. Patients with infectious aetiologies had a median Glasgow Coma Score of 3 as compared to median 4 of patients with autoimmune disease. There are few data on specific post-encephalitic morbidities in the UK and also on outcomes specific to other causes of encephalitis besides herpes simplex. Survivors of encephalitis may have physical, cognitive, emotional and social difficulties [Reference Ramanuj19]. An outcome study in Sweden reported post-encephalitic epilepsy in 24% of survivors of HSE, representing a 60–90-fold greater risk than the general population. Additionally, a 5–15 and 5–11-fold elevated risk of pulmonary embolism and diabetes mellitus, respectively, were reported. An Austrian study described a high frequency of depressive symptoms among survivors of HSE.
It was concerning that despite guidelines, only 53% received aciclovir. Patients receiving a more prolonged course of 14–21 days fell into the category of either undiagnosed or autoimmune encephalitis. One patient had HSE, but HSV DNA may not be detected in the CSF initially if the LP is carried out too early, or after aciclovir has been given. There is evidence that intrathecal antibody testing may be useful in these cases [Reference Stahl17].
In conclusion, it is important to implement standardised first-line investigations to reduce the proportion of cases of unknown aetiology and have standardised diagnostic algorithms. Multidisciplinary work is needed to understand and tackle obstacles in meeting guidelines. In this study, 24% of acute encephalitis was immune-mediated and early recognition of these patients is important as treatment is available and effective, and delayed treatment leads to worse outcomes.






