Introduction
Shiga toxin-producing Escherichia coli O157 (STEC O157) infection causes an estimated 95 000 illnesses and 30 deaths annually in the United States [Reference Scallan1]. Approximately 5–10% of those diagnosed develop haemolytic uremic syndrome (HUS), a severe condition that can lead to long hospitalisations, kidney failure and death [Reference Gould2]. Children may be at greater risk of infection with STEC O157:H7 [Reference Chapman, Cornell and Green3, Reference Lahti4] and have a higher risk of developing severe illness such as HUS [Reference Gould2, Reference Heiman5]. Ruminant animals, such as cows, goats and sheep, are common animal reservoirs for STEC O157:H7 [Reference Gyles6]. Human infections occur through the faecal–oral route and most often from consuming contaminated food; however, outbreaks caused by direct contact with animals or their environments are not uncommon [Reference Heiman5, Reference Ferens and Hovde7–12].
The National Association of State Public Health Veterinarians (NASPHV) and the Centers for Disease Control and Prevention (CDC) created guidelines and recommendations for animal venue operators, exhibitors, public health officials and visitors to prevent the spread of disease in public settings where animals are present [13]. These guidelines are based on results from previous outbreak investigations of human illness in public animal settings, such as petting zoos and agricultural fairs. Guidelines include providing appropriate handwashing stations, displaying signage to explain the risks of disease transmission, and creating unidirectional flow at events to encourage use of handwashing stations and discourage eating or drinking in animal areas [13].
On 27 April 2015, health officials in Whatcom County, Washington were notified of three children with presumptive STEC O157:H7 infection [Reference Curran14]. All had attended a dairy education event held in a barn 20–24 April during school field trips. Whatcom County Health Department (WCHD), the Washington State Department of Health (WADOH) and CDC investigated to determine the magnitude of the outbreak, identify the source of infection, prevent secondary illness transmission and develop recommendations to prevent future outbreaks. We assessed environmental contamination in the barn and risk factors for infection among event attendees.
Methods
Case finding
STEC infection is a notifiable condition in Washington State. Health care providers and health facilities are required to immediately report a case to the local health department without delay. Clinical laboratories are required to report STEC-positive stool results immediately and submit stool specimens or isolates to the WADOH within two business days [15]. Upon detection of a cluster of cases, WCHD notified local laboratories, school nurses, parents and event organisers of the outbreak. WCHD issued health advisories that included outbreak investigation updates, STEC information and advice to the public. WCHD and WADOH interviewed patients with confirmed STEC O157:H7 infection and others who reported diarrhoeal illness regarding their attendance at the dairy education event.
The Enterics Laboratory at the Washington State Public Health Laboratories (WAPHL) conducted STEC O157:H7 confirmatory testing on stool specimens or isolates submitted during the investigation period. The presence of Shiga toxin was determined using the Meridian Biosciences ImmunoStat EHEC Card (Cincinnati, OH). Shiga toxin-producing isolates were biochemically identified as E. coli and subsequently serotyped using the Remel RIM E. coli O157:H7 Latex Test Kit (Lenexa, KS, USA). Confirmed STEC O157:H7 isolates were submitted to the laboratory at the WAPHL for pulsed-field gel electrophoresis (PFGE).
Subsequent analyses, including identification of outbreak strains, were performed and submitted to the CDC PulseNet database. Through PulseNet, the national molecular subtyping laboratory network for foodborne disease surveillance, outbreak strains were identified and defined by two-enzyme (XbaI and BlnI) PFGE pattern combinations. In outbreak investigations, isolates with indistinguishable PFGE patterns are considered more likely to share a common source.
Case definition
For purposes of the outbreak investigation, a confirmed case of STEC O157:H7 infection was defined as laboratory confirmation of infection with one of the outbreak strains or physician-diagnosed HUS in a person with diarrhoeal illness onset during 20 April–1 June, who had attended the dairy event or had close contact with someone who had attended the event. A probable case was defined as diarrhoeal illness in a person with onset during April 20–June 1, who had attended the event or had close contact with someone who attended the event. Confirmed and probable cases were classified as primary cases – patients who attended the event, or secondary cases – patients who had contact with someone who attended the event (e.g. household contact).
Hypothesis generation
Informal, open-ended interviews were conducted in person or by phone with key stakeholders (e.g. fairgrounds and event staff, parents, volunteers, event cleaners, school nurses) to better understand the setup of activities at the dairy education event, previous events at the fairgrounds, cleaning procedures at the fairgrounds, types of attendees, and potential risk factors and sources of infection. Investigators visited the dairy barn and fairgrounds to observe facility layout and handwashing facilities. A telephone survey was administered to first-grade teachers whose classes attended the dairy education event and one high school teacher whose agriculture technology class assisted with event setup and breakdown. Survey questions included: date and time of event attendance, number of students who attended the event, order of activities the class followed, number of students who used hand sanitiser or water and soap at various activities (all, most, some or none), and students’ food consumption by time (e.g. before, during or after the event) and location. The informal interviews and the teacher survey informed the development of the case–control study questionnaire and environmental investigation.
Case–control study
A case–control study was conducted among event attendees to identify risk factors for infection. For the study, a case-patient was defined as a person who attended the dairy education event, and had laboratory-confirmed STEC O157:H7 infection with an outbreak strain, or physician-diagnosed HUS, or diarrhoea (bloody diarrhoea or ⩾3 loose stools/day) within 10 days (i.e. maximum incubation period for E. coli) following event attendance. A control was defined as a person who attended the dairy education event, and who did not develop any signs or symptoms of STEC O157:H7 infection, including diarrhoea, vomiting or abdominal cramps, within 10 days following event attendance. We limited enrolment to one control per household and excluded household contacts of cases and a group of attendees from a local healthcare centre, which had no cases and was not comparable to other groups (due to older age, poorer health status and limited event exposures). We frequency-matched controls to cases 3 : 1 by age category: first-grade student, high school student or adult. First-grade controls were selected from school rosters provided by 18 schools with classes attending the dairy education event. We expected to enrol 40 case-patients and 120 controls. Across the 18 schools, there were approximately 1290 eligible controls. Since school rosters were provided at different times during the investigation, we randomly selected 10% (120/1290 ~ 10%) of first-grade students from each school. Three call attempts on three separate days were made before exclusion for both cases and controls. If controls refused participation or were not reached after three attempts, the next randomly ordered control from the same school was selected. We attempted to interview all of the high school students (n = 23) in the agriculture technology class that assisted with setup and breakdown. Since there were no rosters of adults in attendance, a convenience sample of parents and teachers were selected as controls.
Between 19 and 29 May, federal, state and local health officials administered a questionnaire by phone. For first graders, both parents and children were interviewed. The questionnaire focused on clinical history, hand-to-mouth and hygiene habits, previous animal exposures, participation in event activities, food and beverage consumption, and handwashing.
Environmental investigation
WCHD conducted environmental sampling in the dairy barn 6 and 19 days after the dairy education event. The team collected environmental specimens from each event activity area in the barn. Surfaces, including floors, walls, bleachers, benches and handrails, were swabbed with sterile gauze in a sinusoidal wave to cover approximately 10% of the area. Milking equipment was swabbed using a cotton-tipped applicator. Sawdust shavings, hay and work gloves were sampled. Due to the number of environmental specimens obtained, the laboratory pooled samples (i.e. 2–4 gauzes swabbed from separate surfaces) by event activity station or distinct area in the barn. Specimens were transported in a cooler to the Food and Shellfish Laboratory at the WAPHL for microbiological testing. All samples were enriched overnight in a 37 °C shaking incubator using modified trypticase soy broth. Sample enrichments were plated onto MacConkey agar with sorbitol (SMAC), SMAC with tellurite and cefixime, and Rainbow agar with antibiotics. Suspicious colonies were biochemically identified as E. coli and subsequently serotyped using the Remel RIM E. coli O157:H7 Latex Test Kit. Confirmed STEC O157:H7 isolates were processed for PFGE using XbaI and BlnI restriction enzymes. PFGE patterns of environmental isolates were compared to the outbreak strains defined by patient isolates.
Data analysis
For the teacher survey and case–control study, data were entered into Epi Info version 7.0 and analysed using Stata Version 11.0 (College Station, TX, USA). Descriptive statistics were produced for outbreak linelist data, teacher survey responses and case–control study participant characteristics. Fisher's exact χ 2 tests were used to compare (1) characteristics of first-grade classes with a case-patient to those without a case-patient, and (2) characteristics of case-patients and controls. For the primary analysis of first-grade student case-patients and controls, univariable and multivariable exact logistic regression was performed to compute odds ratios (OR), exact 95% confidence intervals (CI) and two-sided P values. A multivariable model included variables for which P values were ⩽0.05 in univariable analyses; the Hosmer–Lemeshow test was used to assess goodness of fit. OR with a 95% CI that did not include 1.00 were considered statistically significant.
Results
Outbreak investigation
A total of 60 cases of STEC O157:H7 infection (25 confirmed and 35 probable) were identified. Eleven ill people were hospitalised, and six (two high school students, two first-grade students, one 2-year old, one 11-month old) were diagnosed with HUS; none died. Illness onset dates ranged from 24 April to 12 May 2015 (Fig. 1). Ill people ranged in age from <1 year to 47 years (median: 7), and 20 (33%) were female. Forty cases were classified as primary, and 20 were secondary (Fig. 1). Five PFGE pattern combinations were identified from clinical and environmental samples: EXHX01.0047/EXHA26.0071 (pattern A: 21 clinical isolates, nine environmental isolates), EXHX01.2401/EXHA26.0071 (pattern B: two clinical isolates), EXHX01.5960/EXHA26.0071 (pattern C: one clinical isolate), EXHX01.0047/EXHA26.4214 (pattern D: one environmental isolate) and EXHX01.0047/EXHA26.4219 (pattern E: one environmental isolate) (Table 1).
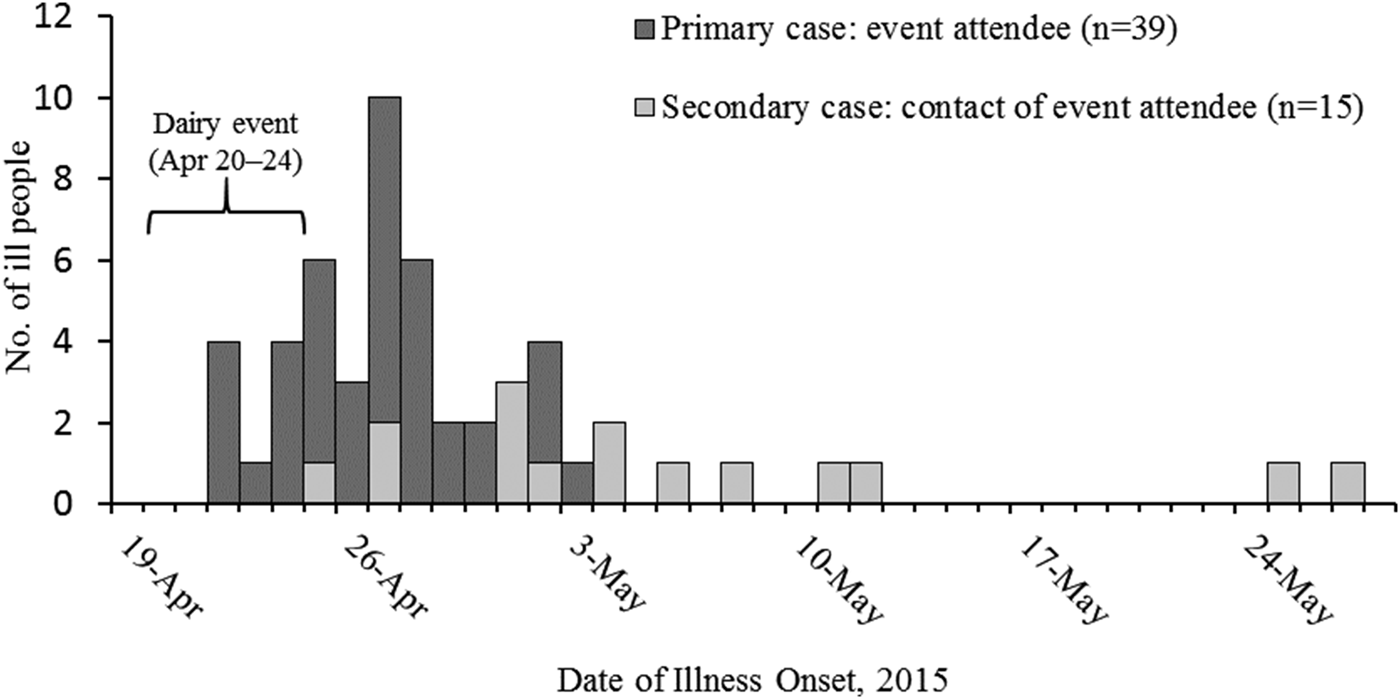
Fig. 1. Number of people (N = 54) infected with the outbreak strains of Escherichia coli O157:H7, by date of illness onset and dairy education event attendance – Whatcom County, Washington, 20 April–1 June 2015*. *Six additional patients (one primary, five secondary) were ill during 20 April–1 June, but exact illness onset dates were unknown.
Table 1. Laboratory STEC O157:H7 test results of clinical isolates and environmental samples collected from barn by collection date
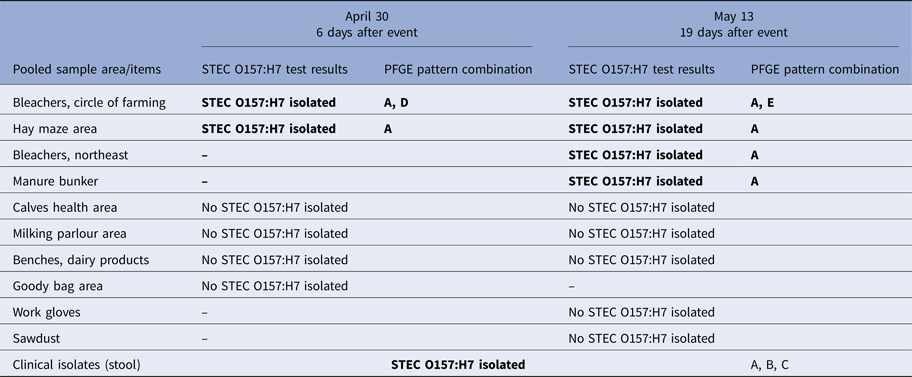
Bold text indicates isolates with PFGE patterns indistinguishable from that of the outbreak strain of STEC O157:H7. Five PFGE pattern combinations were identified from clinical and environmental sources: EXHX01.0047/EXHA26.0071 (pattern A: 21 clinical isolates, nine environmental isolates), EXHX01.2401/EXHA26.0071 (pattern B: two clinical isolates), EXHX01.5960/EXHA26.0071 (pattern C: one clinical isolate), EXHX01.0047/EXHA26.4214 (pattern D: one environmental isolate) and EXHX01.0047/EXHA26.4219 (pattern E: one environmental isolate).
Hypotheses generation
During 21–23 April 2015, approximately 1300 first-grade students attended a dairy education event inside a barn at the fairgrounds. Class groups followed a timed schedule of activities at 12 stations for approximately 3 h, including interactive lectures, a tour of milking facilities, a hay maze, a mobile petting zoo and a wagon ride through the fairgrounds (Fig. 2). Several dairy cows and calves were included in the activities and kept in the barn during the event. Children were supervised while washing their hands with soap and water at one activity station before they received a bottle of pasteurised chocolate milk. Hand sanitiser was given to children before and after the petting zoo and hay maze. Restrooms with handwashing facilities were available near the barn. Goody bags were organised and stored in the barn and given to teachers before departure. Food was not served in the barn or at the fairgrounds; however, some classes brought lunch and ate at the fairgrounds or a nearby park before or after the event.
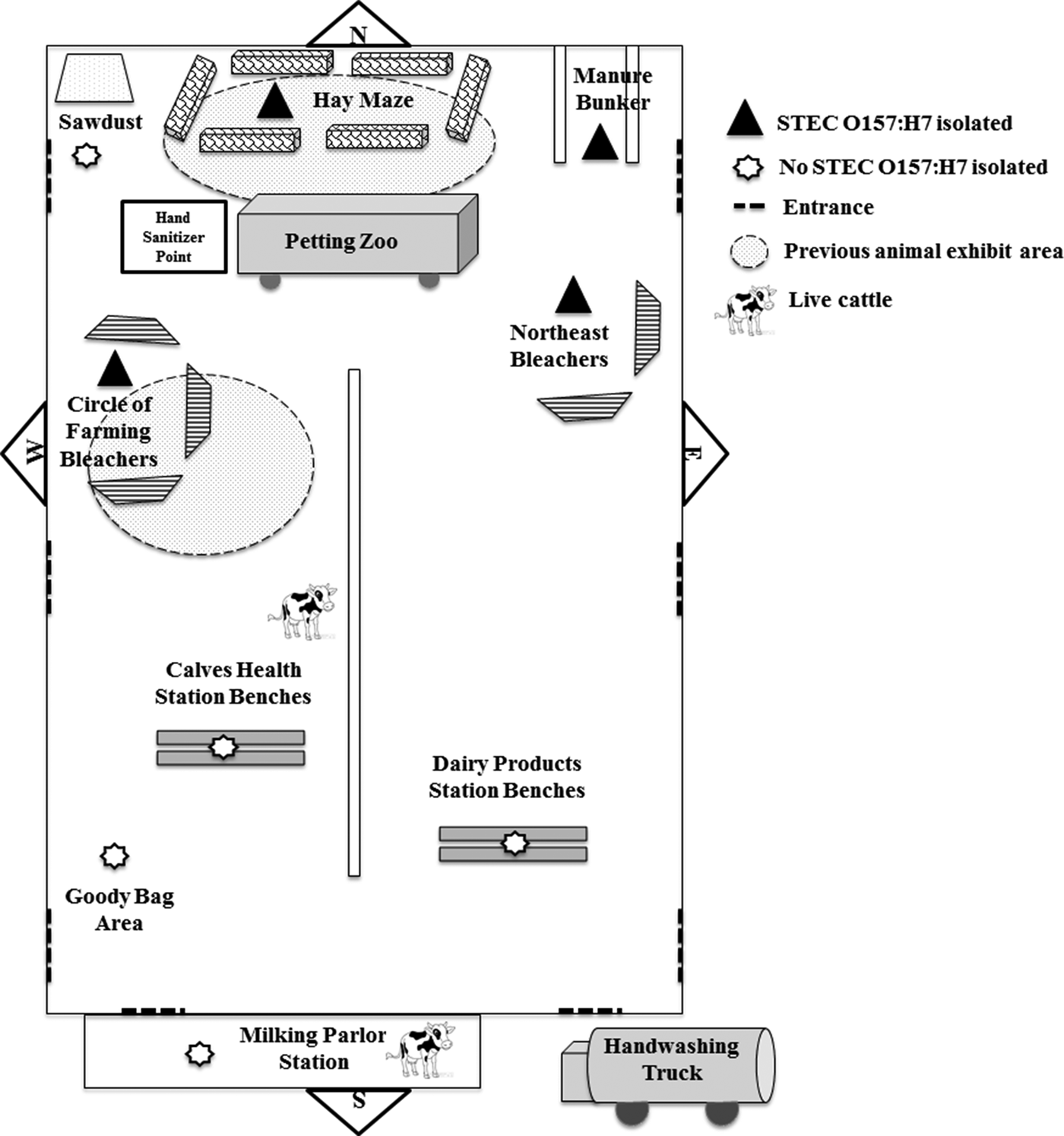
Fig. 2. Barn layout for dairy education event with environmental sampling locations and previous animal exhibit areas. Environmental samples were collected from distinct event activity areas in the barn 6 and 19 days after the dairy education event. The first hay maze sample included hay from four different bales and swabs from the floor and manure bunker exterior wall; the second consisted of swabs from the floor and manure bunker exterior wall. The manure bunker sample was taken from the interior walls and floor. The first and second circles of farming bleachers sample contained swabs of each of the three sets of bleachers. Both northeast bleachers were swabbed. The petting zoo was located inside a trailer and included a miniature donkey, miniature horse, goat, calf, lamb and rabbit. During previous events, animals, including young cattle, were exhibited in the areas shown with shaded circles in the north end of the barn. The bleachers shown at the circle of farming and petting zoo areas were used in the north end of the barn at previous animal exhibits. Items are not drawn to scale.
On 20 and 24 April, high school students assisted with the setup and breakdown of the event by arranging hay bales for the maze and moving cattle panels, bleachers and benches. Hay bales had been delivered on pallets; some had been used at a previous event at the fairgrounds. Work gloves were available and used. Food (i.e. donuts and cookies) and beverages were served inside the barn on the bleachers after both setup and breakdown.
According to interviews, animals, including cattle, had been exhibited in the barn 10 days before the dairy education event. After the event, tractors, scrapers and leaf blowers were used to move manure and other waste to a manure bunker at the north end of the barn. The manure bunker was only accessible from inside the barn. The location of several activity stations at the dairy education event coincided with the locations where cattle were shown at these previous events (Fig. 2). On 5 May, 5 days after the first environmental samples were collected but 8 days before the second set of environmental samples were collected, the barn was swept and cleaned using a backpack sprayer with a water and bleach solution.
One high school teacher and 52 (93%) of 56 first-grade teachers whose classes attended the dairy education event completed the survey. Eleven classes (21%) attended the event on the first day, 20 (38%) on the second day and 21 (40%) on the third day. Thirty-two classes attended a morning session (62%), and the remaining 20 (38%) during the afternoon. Several classes missed one or more of the activities. Most teachers reported that ‘all’ of their students washed their hands at the handwashing truck (92%) and sanitised their hands before (79%) and after (72%) interacting with animals at the mobile petting zoo. A lower proportion of teachers reported that ‘all’ of their students washed their hands before eating lunch (60%). Twenty-one (40%) classes ate lunch at the fairgrounds, 27 (52%) in a classroom or school cafeteria, and four (8%) at a nearby park.
Among 51 first-grade teachers interviewed with available data on whether there was at least one case in the class, the proportion of classes with a case differed significantly by event attendance date (P < 0.01) (Table 2). Over half of classes with a case (10/19, 53%) attended the first day (e.g. Tuesday, 21 April), while only one class without a case (1/32, 3%) attended then. Among 11 classes that attended the dairy event on the first day, 10 (91%) had at least one case in the class. Classes with a case did not significantly differ from classes without a case by eating and handwashing behaviours reported by teachers (Table 2).
Table 2. Selected characteristics reported by teachers of first-grade classes with a case, as compared with classes without a case
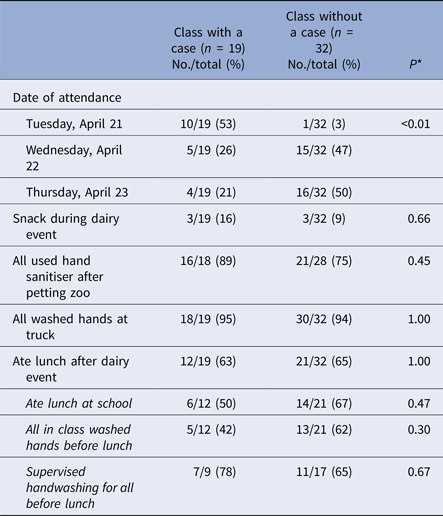
* Fisher's exact test.
Case–control study
A total of 44 ill event attendees were contacted for participation in the study: 11 (25%) were not reached, two (5%) declined and 31 (70%) completed the questionnaire, of whom four failed to meet the inclusion criteria (i.e. ⩾3 loose stools/day). Among 27 eligible case-patients enrolled in the study, there were 23 first-grade students included in the primary analysis, three high school students and one adult. A total of 198 controls were selected for interview: 94 (47%) were interviewed, 61 (31%) were unable to be reached after three attempts, 18 (9%) had incorrect or missing contact information, 14 (7%) were ineligible and 11 (6%) refused. Six controls were considered ineligible after the interview. Eighty-eight eligible controls were enrolled in the case–control study: 75 first-grade students included in the primary analysis, eight high school students and five adults.
Overall, case-patients did not significantly differ from controls in terms of gender and race. The majority of case-patients (23/27, 85%) and controls (75/88, 85%) were first-grade students. Most case-patients and controls were white (70% and 76%, respectively); one case-patient and one control were American Indian/Alaskan Native (4% and 1%), three controls (3%) were black/African American, three controls were Asian (3%), and six case-patients and 13 controls identified with another race (22% and 15%). One-third of case-patients (9/27, 33%) and 14% of controls (12/88, 14%) were Hispanic.
Case-patients had a median illness duration of 7 days (range 1–15 days) and a median of two visits to a health professional (range 0–10 visits). The majority of case-patients (26/27, 96%) reported abdominal cramping; 18 (67%) had bloody diarrhoea; 17 (63%) had nausea and fever; 12 (44%) had vomiting and 3 (11%) had constipation. Four (15%) were diagnosed with HUS.
Of the high school students, all case-patients and controls (n = 11, 100%) were non-Hispanic and white. All reported touching hay bales and having snacks inside the barn. None of the high school case-patients (0/3, 0%) reported washing or sanitising their hands before eating, while a quarter of high school controls (2/8, 25%) used hand sanitiser before eating snacks in the barn. Six adult women were interviewed: one case-patient and five controls. The adult case reported having lunch at the fairgrounds after attending the event Tuesday morning (April 21) without handwashing. While two adult controls also attended Tuesday morning; one attended Wednesday morning and two on Wednesday afternoon. The only adult control with information reported handwashing before lunch.
In the primary analysis of the case–control study, first-grade case-patients were significantly more likely than first-grade controls to be male (OR 2.97; 95% CI 1.02–9.09), Hispanic (OR 3.74; 95% CI 1.12–12.06) and have attended the event on the first day (e.g. Tuesday, 21 April) (OR 3.38; 95% CI 1.07–10.74) (Table 3). Among first graders, 26% of case-patients reported ‘always biting nails’ compared with 5% of controls (OR 6.26; 95% CI 1.29–32.90, P = 0.01). First-grade case-patients were significantly less likely to report washing or sanitising their hands before eating lunch compared with controls (18% vs. 64%; OR 0.22; 95% CI 0.05–0.79, P = 0.01). Previous farm animal exposure, visiting the petting zoo, having contact with animals or cows at the event, eating lunch at the fairgrounds, and eating or drinking in the barn were not associated with illness (Table 3).
Table 3. Odds ratios for selected characteristics of first-grade case-patients, as compared with controls
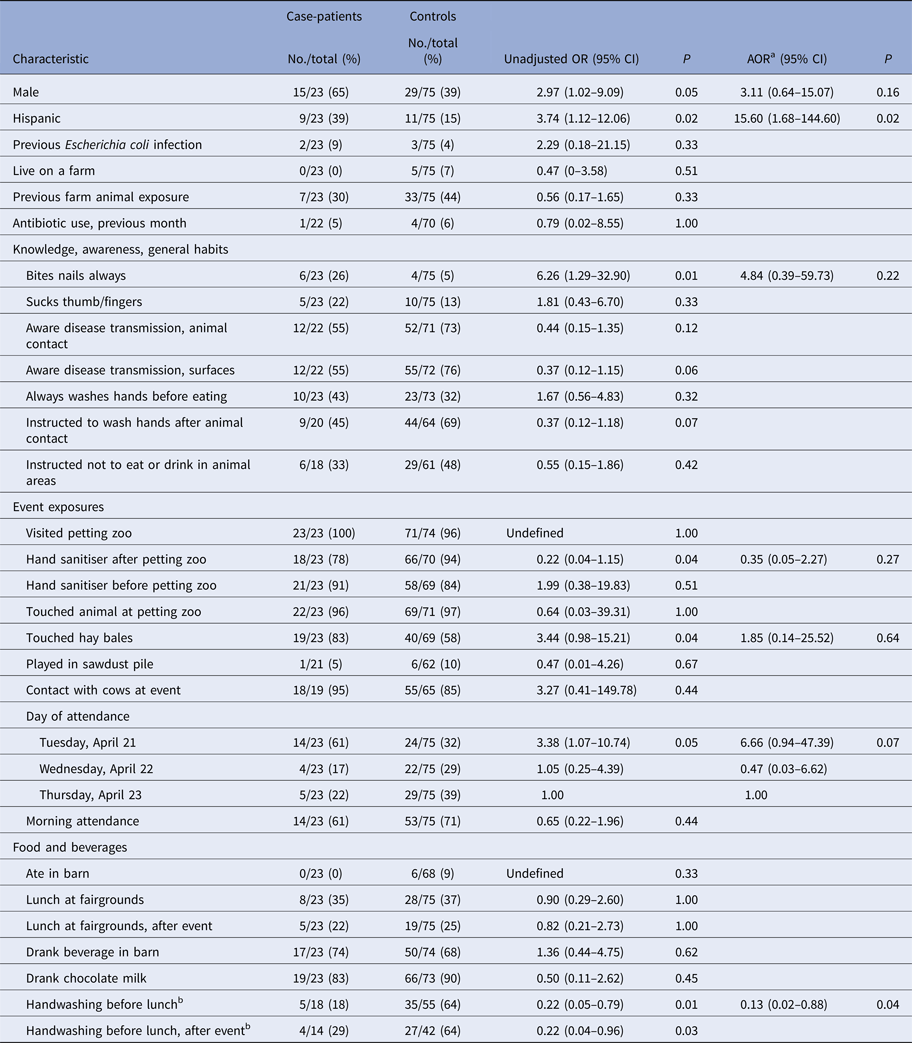
a Adjusted model includes gender, ethnicity, nail biting, hand sanitiser use after petting zoo, touching hay bales, day of attendance and handwashing before lunch.
b Handwashing with soap and water or hand sanitiser.
No, number; OR, odds ratio; CI, confidence interval; P, P value; AOR, adjusted odds ratio.
The final multivariable model included gender, ethnicity, nail biting, hand sanitiser use after petting zoo, touching hay bales, day of attendance and handwashing before lunch. Washing hands with soap and water or hand sanitiser before lunch [adjusted OR (AOR) 0.13; 95% CI 0.02–0.88, P = 0.04) and being Hispanic (AOR 15.60; 95% CI 1.68–144.60, P = 0.02) remained significantly associated with illness. A multivariable model with variables with P < 0.2 in univariable analyses included fewer observations and yielded similar results.
Environmental investigation
A total of 15 pooled environmental samples were collected from the barn and tested, including six samples collected 6 days after the dairy education event and nine samples collected 19 days after the event (Table 1). STEC O157:H7 was isolated from samples from four areas or items in the barn (the bleachers at the circle of farming station, the northeast bleachers, the hay maze area and the manure bunker) and yielded isolates with PFGE patterns which were indistinguishable from those of the outbreak strain of STEC O157:H7 (Fig. 2). All of these samples had been collected from the north end of the barn (Fig. 2).
Discussion
This investigation provided epidemiological, laboratory and environmental evidence for a large point-source outbreak of STEC O157:H7 infections from exposure to a contaminated barn with secondary transmission among contacts of dairy event attendees. In the case–control study among first-grade students, handwashing before lunch was protective, while general nail biting habits were associated with illness. The majority of first-grade students ate lunch after attending the event; according to both self-report and teacher accounts, not all washed their hands before eating, despite access to handwashing facilities at the fairgrounds and school. All high school case-patients interviewed ate in the barn after setup without washing or sanitising their hands. Eating or engaging in hand-to-mouth behaviour after spending time in the barn, where attendees had hand contact with contaminated surfaces (e.g. bleachers), likely explains the mode of transmission for attendees who became ill. In the case–control study also, Hispanic ethnicity was associated with illness among first-grade students which may reflect a true relationship (e.g. due to language barriers), residual confounding (e.g. classes with different demographics had different exposures that were not captured by the questionnaire), lower response rates among Hispanic controls (data not available to test) or a spurious association.
Outbreak strains of STEC O157:H7 were identified in several areas in the barn after the event and after venue cleaning, indicating widespread contamination and persistence of the bacteria in the environment. The barn was likely contaminated from previous animal exhibitions with cattle. Healthy cattle, a major source of STEC O157:H7 infections in humans, can shed without signs of illness and may shed more under stressful circumstances [Reference Ferens and Hovde7]. E. coli O157 can survive in the environment (including dust) for up to 42 weeks [Reference Varma16] and endure various efforts to eradicate the bacteria [Reference Durso17]. While the methods used in cleaning the facility (e.g. use of leaf blowers) might have contributed to an increased risk for infection; previous research underscores the difficulty in decontaminating animal environments [Reference Durso17]. Similar to previous outbreaks of E. coli O157 infections associated with exposure to animal environments [Reference Crump18] or contaminated buildings, [Reference Varma16] we identified handwashing as protective against infection.
One-third of outbreak cases identified were secondary, exposed through contact with a relative, close contact or possibly a fomite (e.g. shoes, article of clothing, goody bag) – indicating a high rate of secondary transmission and important opportunities for prevention. A statistical review of E. coli O157 outbreaks in Europe and the US estimated that 20% of outbreak-related cases result from secondary spread, with higher rates of secondary transmission in outbreaks primarily affecting children [Reference Snedeker19]. Similarly, a 10-year review of E. coli O157 surveillance data in Scotland found that 11% of all infections were attributable to secondary transmission [Reference Locking20]. The high rate of secondary transmission in this outbreak might be explained by the young age of the primary and secondary cases, many of whom lived in the same household or shared a caregiver. Throughout the investigation, WCHD, WADOH and CDC provided public health messages through press releases, emphasising precautions to take to prevent secondary illnesses, including handwashing and staying home from school while diarrhoea persists. Letters in English and Spanish were given to schools to share with teachers and parents to inform them of the outbreak, the signs and symptoms of E. coli infection, and prevention and treatment measures.
School field trips that involve animals or animal environments offer education and entertainment to children but also pose a known health risk [Reference Lee and Greig21, Reference Lange22]. Certain factors might increase risk for infection, especially in children: lack of awareness of the risk of disease transmission in animal settings, more frequent contact with animals and the environment, inadequate handwashing, and hand-to-mouth behaviours or activities (e.g. eating) [13, Reference McMillian23]. According to a recent review, animal contact, through school field trips or class activities, contributed to 12% of gastrointestinal outbreaks in schools [Reference Lee and Greig21]. Recognising the beneficial value of events that involve animals and animal contact, NASPHV, in collaboration with federal partners including CDC, published recommendations to minimise the risk of disease associated with animals in public settings [13]. These guidelines emphasise: (1) handwashing with clean, running water and soap immediately after exiting animal areas, after removing soiled clothing or shoes, and before eating or drinking; (2) consumption of food and beverage only in non-animal areas; (3) designing facilities and event layout to minimise risk from animal contact and promote handwashing; (4) following standard protocols for cleaning and disinfection; and (5) informing visitors of risk of disease transmission from animals and animal environments and promoting prevention behaviours through age-appropriate materials and activities [13]. Handwashing with soap and water is the preferred method to reduce the number of germs on hands; alcohol-based hand sanitisers can also reduce germs, but are less effective when hands are visibly dirty or greasy [24].
Limitations
For the teacher survey, classrooms were categorised as having a case based on the investigation linelist which may have resulted in misclassification of classroom case status. The case–control study relied on self-reported data, which might have introduced social desirability bias and recall bias and resulted in misclassification of exposures. For example, case-patients and their parents may have had better recall of the dairy education event activities and been more likely to report risk behaviours, possibly inflating the association between lack of handwashing and infection. Reports of past behaviours may also be less reliable from young children. A small sample in the case–control study limited the size of the multivariable model and the ability to examine more complex relationships between illness and risk factors. Many of the event activities investigated as possible exposures were attended by the majority or all, making it difficult to detect increased risk by activity. As a result of limited resources for the environmental investigation, it was not possible to individually test every area of the barn that was sampled. Negative results do not rule out the possibility of contamination in other areas of the barn.
Conclusions
This investigation of a large outbreak of STEC O157:H7 infections following a school field trip highlights the often overlooked risk of infection through exposure to animal environments, even those without direct animal contact. Infection prevention measures are critical to implement at events held in venues with animals or where animals have been, especially when children or other vulnerable populations are present. Although it might not be possible to completely disinfect barns and areas where animals have been kept, established guidance to prevent animal-associated disease through cleaning, disinfection, facility design and health promotion should be adopted [13]. Barns or animal environments should be considered contaminated even when animals (and animal waste) are not present; food and beverages should not be served in these locations. Event attendees should be reminded to always wash their hands with soap and clean running water, and dry with clean towels immediately upon exiting animal areas or areas where animals have been kept previously, after removing soiled clothing or shoes, and before eating or drinking [24]. Increased education and encouragement of infection prevention measures such as handwashing can prevent illness and future outbreaks.








