INTRODUCTION
Hepatitis A virus (HAV) is a ubiquitous enteric picornavirus responsible for the acute disease hepatitis A. It is spread by contaminated water and food and is easily transmitted from person to person via the faecal–oral route. In the early 20th century hepatitis A was common in the UK but with improved sanitation it is now relatively infrequent. Since 1987 hepatitis A has been a notifiable disease and has been on the decline since the 1990s [Reference Crowcroft1]; the number of cases decreased by 90% between 1992 and 2004 (from 6762 to 669) [2]. In parallel the reported incidence of hepatitis A in the European Union (EU) has decreased from 15·1/100 000 in 1996 to 3·9/100 000 in 2006 [Reference Payne and Coulombier3]. Outbreaks within the EU are usually caused by importation of the virus from an endemic area, known as a ‘seeding event’ [Reference Payne and Coulombier3]; the ‘seed’ most commonly being a previously susceptible HAV-infected traveller. Onward transmission from the ‘seeding event’ is usually contained but can lead to outbreaks and onward transmission in the community if it enters into settings/cohorts where the potential for faecal–oral spread exists. This has been described in childcare centres and nurseries [Reference de Paula4, Reference Hauri5], primary schools [Reference Leoni6], men who have sex with men [Reference Stene-Johansen7, Reference Tortajada8] and injecting drug users [Reference Grinde9, Reference Tjon10]. Transmission and spread into the community can now more easily occur due to the increasing susceptibility of the population and the absence of universal immunization in the UK.
Over the last decade the majority of outbreaks reported in England and Wales have been related to injecting drug use [Reference O'Donovan11–Reference Ngui17] although outbreaks in the community [Reference Roberts and Palmer18] and in men who have sex with men [19] have also been recognized. The last reported outbreak in a school was in 2006 where nine cases were notified associated with a primary school in the Liverpool area where the source was never identified [Reference Taylor-Robinson20]. Here we describe an outbreak of hepatitis A linked to two schools and the measures taken to control it.
Chronology of the hepatitis A cases
All cases were reported to the Hampshire and Isle of Wight Health Protection Unit (HIOW HPU).
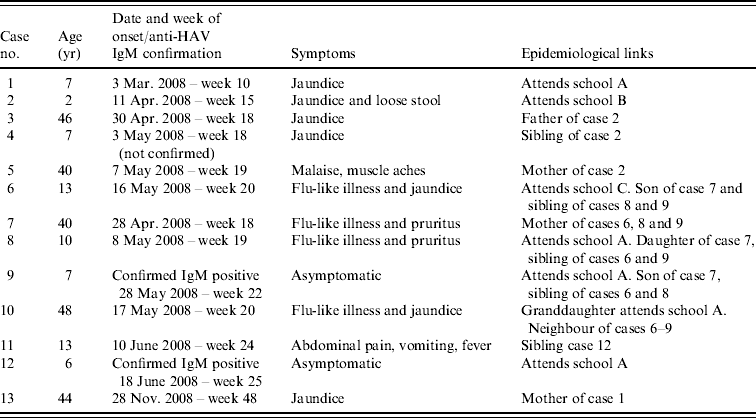
On 3 March 2008 hepatitis A was diagnosed in a 7-year-old schoolchild who developed jaundice (case 1). The pupil attended a primary school (school A) in the Winchester area and was excluded from school for 7 days from the date of onset of jaundice. All the household contacts (mother and father) were immunized with a single dose of vaccine within 7 days of onset of jaundice in the index case in accordance with guidelines [21].
Between 11 April 2008 and 7 May 2008 a further four cases of hepatitis A were diagnosed in another family. The first case in this family was a 2-year-old child (case 2, onset of jaundice 11 April) who attended a nursery school (school B) 1·6 miles away from school A, attended by case 1 between which no epidemiological link was known at the time or subsequently discovered. Cases 3 (onset 30 April), 4 (onset 3 May) and 5 (onset 7 May) were the father, sibling and mother of case 2, respectively. Cases 3–5 were vaccinated after case 2 was diagnosed but the onset of their disease suggests that they were already incubating the virus at the time of vaccination.
On 16 May 2008 case 6 developed jaundice, this child attended a special needs school (school C) in the Winchester area. Cases 6 and 1 shared the same General Practitioner which led to the discovery that case 6 had a sibling in the same class as case 1 in school A. The link with the primary school led to the establishment of an outbreak committee. The household members (mother and two siblings) of case 6 were screened prior to immunization and all three were found to be anti-HAV IgM seropositive. The mother (case 7), had reported mild flu-like symptoms at the end of April. The siblings aged 10 (case 8, onset 8 May) and 7 years (case 9, asymptomatic) attended school A with case 9 being in the same class as case 1. Case 9 had an asymptomatic infection and it was initially assumed that he introduced the virus into this household; however, it was more likely to have been his sibling case 8 as she had nearly cleared the virus at the time of testing. The family had been unaware of any child having had hepatitis A in school A until this time.
Case 10 was a 48-year-old female who developed jaundice on 17 May. This case was connected to the outbreak by her granddaughter who was in the same class as case 1 and her neighbours, cases 6–9. On screening, the granddaughter was found to be negative for anti-HAV IgM, therefore making it unlikely that she had a recent infection and was the source of infection for case 10; however, cases 8 and 9 would often play with her at the home of case 10. Case 10 was a healthcare worker (HCW) on a maternity unit at a hospital in Winchester. She had been non-specifically unwell for nearly 2 weeks before developing jaundice and continued to work until this time, this led to the recall and screening of 42 babies and is detailed later.
On 10 June 2008, there was a further case diagnosed (case 11, aged 13 years) who had a sibling that attended school A. This sibling reported no symptoms, but on screening was found to be anti-HAV IgM seropositive indicating recent infection (case 12), she had not been vaccinated although mass vaccination of school A had begun on 4 June 2008.
No further cases were notified after June 2008 and the outbreak was closed only to be re-opened on 23 December 2008 after the mother of case 1 was admitted to hospital with jaundice and was found to be anti-HAV IgM seropositive on 30 November 2008 (case 13); she had previously been immunized in March 2008. The information on the cases is summarized in Table 1.
Table 1. Cases notified to the Hampshire and Isle of Wight Health Protection Unit from the Winchester area
School A=primary school; school B=nursery school; school C=special needs school.
Outbreak control
The HPUs are responsible for the management of outbreaks of communicable disease within the geographical area they serve. They coordinate agreed actions and ensure a coherent and timely response from all stakeholders involved. Control of the outbreak centred on identifying the cases and immunizing close contacts which in this case resulted in vaccine being given to 259 pupils and 124 staff: mass vaccination in the schools began on 4 June 2008. Figure 1 demonstrates the potential spread of the virus.
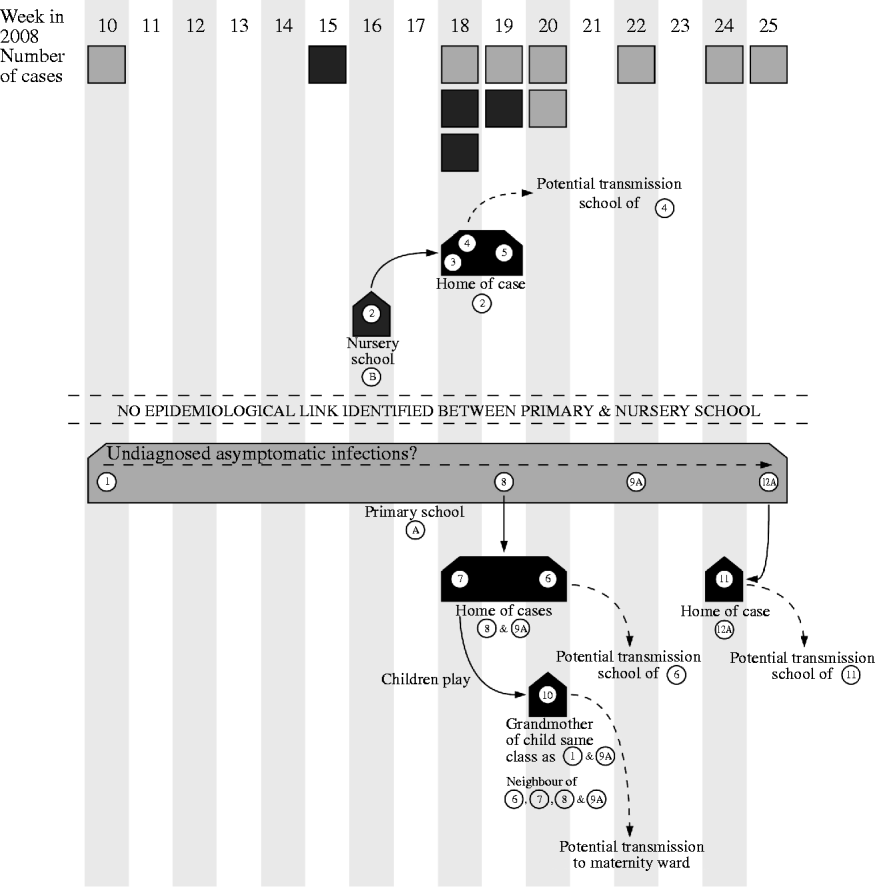
Fig. 1. Diagram showing the weeks that cases were notified to the health protection unit and the movements of the virus during the outbreak. A, Asymptomatic case. ![]() , Case related to primary school;
, Case related to primary school; ![]() , case related to nursery school.
, case related to nursery school.
School inspections
Inspection of the schools was performed by the Children's Services Health and Safety Officers, Environmental Health Officers and the HPU. There had been no reported increase in sickness levels in staff or pupils at any of the schools involved. The inspection of the primary school (school A) attended by cases 1, 8, 9 and 12 raised concerns regarding the toilet facilities for the children and an uncovered water tank. While the uncovered water tank was not considered to be the source in this outbreak, the children's toilets were considered substandard and lacked the basic hand-washing amenities of warm water and liquid soap. At the special needs school (school C) attended by case 6, breaches in infection control in the sluice facilities and the food technology room were noted but neither was considered to be able to facilitate transmission of the virus. Inspection of the nursery (school B) attended by case 2 did not uncover any problems that could facilitate transmission of HAV.
The hospital
Case 10 was a maternity support worker in the perinatal ward at the Royal Hampshire County Hospital (RHCH), Winchester. Her work involved supporting mothers in the postnatal period. She assisted mothers in caring for their babies and with breastfeeding, but did not specifically prepare feeds for babies. She was considered to be linked to the school outbreak by virtue of both her granddaughter who was in the same class as case 1 and by her neighbours who were the family of cases 6–9.
Risk assessment by the members of the outbreak committee considered that the week prior to the onset of jaundice was the most likely period in which potential transmission could have occurred. Sixty-seven women gave birth in this week.
The risk to the mothers was deemed low and immunization was not felt to be indicated as their contact with the HCW was limited. The risk to the neonates was also deemed to be low and while hepatitis A in neonates is mild in nature concern was raised regarding possible tertiary spread into the community from asymptomatic but infected babies. As the vaccine is not licensed for use in neonates, screening for infection by dried blood spots (DBS) taken by heel prick was offered to establish/exclude any asymptomatic infection in the neonates. None were identified.
Virological investigation
All serology was performed at the RHCH, Winchester. HAV RNA detection and genotyping in identified cases and processing of DBS was performed at the Virus Reference Department (VRD) of the Health Protection Agency (HPA).
HAV RNA detection and genotyping from sera
Twelve anti-HAV IgM-positive samples were submitted for HAV RNA by qualitative reverse transcription–polymerase chain reaction (RT–PCR) for genotyping, nine of which were also tested by real time RT–PCR. Nucleic acid was extracted from 200 μl of serum using the QIAamp Ultrasens Virus kit (Qiagen, UK) according to the manufacturer's instructions; RNase-free water was used as negative control and HAV strain HM175 (NIBSC) was used as the positive control. Real-time detection of HAV RNA was performed using the Artus HAV RT–PCR kit (Qiagen) according to the manufacturer's instructions. This assay was employed as it allowed rapid HAV RNA detection and viral load assessment for each sample (Table 2) and was particularly informative in cases 8 and 9. The latter was asymptomatic and thought to have introduced the virus into the household but as his sibling (case 8) had almost cleared her virus at the time of testing it was more likely that she was the source for the family. The qualitative RT–PCR, amplification of a 356-bp fragment covering the VP1/2PA junction, and genotyping was performed as previously described [Reference Ngui17]. Eleven of the HAV IgM-positive samples had detectable HAV RNA and it was possible to genotype 10 of these samples by sequencing. One sample containing HAV RNA detectable by real-time RT–PCR was inhibitory in the qualitative RT–PCR and could not be sequenced. The genotype of the virus in all cases was genotype IB and was unlike any previously sequenced in the VRD. The results for all molecular testing are summarized in Table 2. Comparison to the BLAST database (http://www.ncbi.nlm.nih.gov/blast/) showed that the strain was found to have a homology of 99% to a strain isolated from hirsute clams (Genbank accession no. DQ452802) which is known to cause rolling transmission and outbreaks of HAV infection in Mozambique [Reference Nenonen22].
Table 2. Summary of the HAV reverse transcription–polymerase chain reaction (RT–PCR) and genotyping results
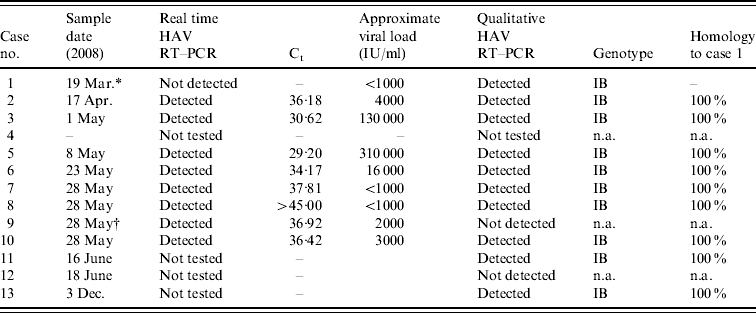
Ct, Cycle threshold; n.a., not applicable.
* Only 30 μl of sample available for nucleic acid extraction.
† Sample inhibitory to PCR.
Anti-HAV IgM and HAV RNA detection from DBS
DBS from 42 babies (aged between 26 and 39 days, mean 30 days, at time of sampling) were sent to VRD for testing. In the absence of DBS from known cases, positive control DBS were created from the HAV strain HM175 (WHO International Standard, NIBSC) and serum samples from acute cases known to be both HAV RNA and anti-HAV IgM positive. Citrate-treated whole blood was taken from a HAV seronegative, blood group O, rhesus-negative individual, the plasma of which was removed and replaced by an equal volume of control serum and the red blood cells then re-suspended; the final HAV RNA concentration of the DBS created from the WHO International Standard being 4×104 IU/ml of whole blood. Twenty-five microlitres of the blood were pipetted on to each spot of a Guthrie card (Schleicher & Schuell 903 paper) and left to dry at room temperature. Negative control DBS were made from HAV RNA and anti-HAV negative whole blood.
For HAV RNA detection a 6-mm spot was punched out from each DBS and digested overnight at 56°C in ATL buffer and proteinase K (Qiagen). The digests were then extracted on the BioRobot Universal System using the QIAamp Virus BioRobot MDx kit (Qiagen). Real-time detection of HAV RNA was performed using the Artus HAV RT–PCR kit (Qiagen) according to the manufacturer's instructions and has a lower limit of detection of 56 IU/ml. In addition to the extraction control provided in the RT–PCR kit, detection of human pyruvate dehydrogenase was used to confirm efficient extraction of the DBS (courtesy of Dr Philip Tuke).
For anti-HAV IgM detection a 6-mm spot was punched out from each DBS and eluted overnight at 4°C in 200 μl elution buffer (PBS/0·05% Tween-20/0·08% sodium azide). Anti-HAV IgM was tested on 20 μl of eluate using the Bioelisa HAV IgM kit (Biokit, UK) according to the manufacturer's instructions; the amount of serum in 20 μl eluate was estimated to be equivalent to the amount of serum required for the test.
DISCUSSION
No source was identified for either cases 1 or 2. This is not surprising as it is estimated that 50% of cases have no identifiable source [Reference Mbithi23, Reference Staes24] which is probably a result of contact with an asymptomatic source. The possibility of local food stores or water supplies in the area being the source was considered, but this was deemed unlikely due to the number of cases. Although there was no evidence of a common source or an epidemiological link between cases 1 and 2 the sequence identity points to the cases being linked. The genetic stability of the HAV genome is such that the sequence of the virus remains unchanged throughout an outbreak [Reference Bower25], therefore establishing sequence identity can enable apparently disparate cases to be linked.
The inability to identify a common source may be due to the fact that the virus had been circulating in asymptomatic cases for some time in the school and that case 1 was not the index case. The epidemiology suggests person-to-person spread at the primary school and within families and the origin may have been an imported travel-related case, particularly given the homology to a Mozambique strain. It is not known if there were any other asymptomatic cases within the school as the only children in the school screened for HAV IgM prior to vaccination were those directly related to a symptomatic case. At least two children (cases 9 and 12) were identified with asymptomatic infections, one of which (case 12) had undetectable virus by the time their sibling became jaundiced. Primary schools, nurseries and daycare centres provide excellent hubs for amplification of HAV transmission because young children are rarely symptomatic and of an age which may have less regard for personal hygiene. Faecal shedding of the virus can be detected as early as 4 days after new world monkeys were infected intravenously [Reference Lemon26]. Infectious virus is also shed in the saliva during incubation and early acute phase of the disease [Reference Mackiewicz27]. Furthermore, in a study of 195 IgM-seronegative children in a school with an ongoing outbreak, 13% were found to have detectable HAV RNA in their serum [Reference de Paula28]. All these observations indicate that a considerable burden of infection may be in existence before an index case presents in young children.
During the inspections of the schools, the toilets in school A were highlighted as being substandard and it has been shown that substandard toilet facilities can contribute to spread of HAV [Reference Rajaratnam29]. This facility had been due for refurbishment but planned work had been suspended due to a recent arson attack. HAV is an extremely robust non-enveloped virus able to withstand low pH and can still be infectious on fingertips after 4 h [Reference Mbithi23]. HAV is easily transferred to hard surfaces where it can survive for prolonged periods of time and transfer is most efficient when the inoculum is wet. HAV can also be transferred to food from contaminated surfaces by non-infected individuals [Reference Schwarz30]. HAV can survive for several days on refrigerated and room temperature food particular under humid conditions. In addition snap freezing of food effectively preserves infectivity [Reference Butot, Putallaz and Sánchez31].
The mother (case 13) of case 1 contracted HAV 9 months after her daughter and 5 months after the last known case in the outbreak, despite having being immunized early in the outbreak. She received a single dose of vaccine in accordance with guidelines; she was not tested for serological response to the vaccine. There is a possibility of a prolonged incubation of the virus due to the single dose of vaccine but this is unlikely given the length of time since her daughter was infected. In the first published study where a single dose of vaccine was used to try and control large outbreaks of HAV it was found that 8–10% of individuals failed to produce a detectable response after a single dose [Reference McMahon32]. Failure of the vaccine after a complete course is rare with only a handful of cases reported [Reference Elliott, Kunze and Torresi33, Reference Bonanni34]. The likelihood that she became infected due to prolonged shedding in her daughter is remote: children have been known to excrete HAV RNA at low levels for up to 10 weeks after onset of symptoms [Reference Robertson35] but stool containing low levels of PCR-only detectable virus does not infect tamarins suggesting the risk of infection is low [Reference Polish36], in addition she was infected more than 10 weeks after her daughter became symptomatic. Residual environmental or possibly even frozen food contamination from the earlier outbreak may have been the source of her infection, or unrecognized asymptomatic cases in the community.
This outbreak undoubtedly had the potential to spread into three schools, one of which was a special needs school, and a maternity ward. Rapid action was required, and delivered for both the special needs school (school C) and the maternity ward. The students of school C had a wide range of physical abilities so despite only one reported case of hepatitis A at this school, the additional risk due to the special requirements of the students and the nursing care provided by the teachers warranted that all the children and all staff with direct personal contact with the students were offered vaccine. This approach has been proven to be extremely effective in controlling outbreaks in this type of school [Reference Ang37]. With the maternity ward there were concerns about potential tertiary spread from infected babies, rather than infection in the neonates themselves, to other family members thus extending the outbreak. Although the vaccine has proven to be immunogenic in neonates the long-term response is reduced by the presence of circulating maternal antibody [Reference Bell38]. In addition, as the vaccine was not licensed for this age group and would have limited benefit to the neonates themselves, it was decided to determine if any of the neonates were infected. DBS have already been shown to be useful for detection of anti-HAV in adolescents [Reference Gil39]. Testing by DBS was chosen in preference to oral fluid, for a number of reasons: first, neonates do not have gingival crevices as they are usually edentulous and as a consequence have little or no gingival crevicular fluid which facilitates antibody secretion into the oral cavity [Reference Parry40]; second, there was no rapid access in VRD to salivary testing for anti-HAV; and third, the DBS could be used for HAV RNA detection which would be present before anti-HAV IgM during the incubation phase of the infection. This approach was taken, although not previously used on DBS taken from neonates, due to the sensitivity of the HAV RNA assay (56 IU/ml) and the input volume of sera eluted from the DBS punch equating to the amount of sera normally added to the anti-HAV IgM assay. The assay controls and the control material created for the testing performed as expected and none of the neonates were found to be infected.
CONCLUSION
Further spread of the outbreak was limited by prompt control measures including the vaccination of contacts. The collaborative work between the Centre for Infections, Environmental Health Officers, Health and Safety Officers, acute trust, and the primary care trust made this a successful multi-agency response. However, this outbreak has highlighted that a single sporadic case without any clear travel history may be the forerunner of an extensive community outbreak, suggesting that transmission had already occurred. This outbreak affected three schools and associated families and it is likely that undiagnosed and asymptomatic cases helped potentiate the spread over a period of time. Detailed epidemiological investigation combined with rapid action in identifying risk groups and controlling transmission was required to limit further extension of the outbreak.
ACKNOWLEDGEMENTS
Preparation of this report was coordinated by the authors in collaboration with The 2008 Winchester HAV Outbreak Team.
APPENDIX. The 2008 Winchester HAV Outbreak Team
K. Balogun, Centre for Infections, Health Protection Agency (CfI HPA); D. Bainbridge, Hampshire County Council (HCC); H. Barrett, HCC; L. Booth, Hampshire and Isle of Wight Health Protection Unit (HIOW HPU); T. Cash, HPA; S. Goodwin, Royal Hampshire County Hospital (RHCH); K. Gosling, Winchester District Council (WDC); L. Halfpenny, RHCH; S. Harriman, Hampshire Primary Care Trust (PCT); S. Jamarani, Virus Reference Department (VRD), HPA; J. Maund, HIOW HPU; F. Neely, HIOW HPU; M. O'Brien, Hampshire PCT; R. Parnaby, RHCH; K. Rowles, HIOW HPU; E. Sgroe, VRD, HPA; P. Shobbrook, RHCH; A. Spencer, WDC; G. Taylor, Hampshire PCT; P. Tuke, National Blood Service; N. Wallis, VRD, HPA.
DECLARATION OF INTEREST
None.




