INTRODUCTION
Salmonella enterica is a common cause of enteric infection in developing countries [Reference Crump, Luby and Mintz1]. In South East Asia, the most common serogroups are S. Typhi and S. Paratyphi A (SPA) [Reference Maskey2, Reference Vollaard3]. In recent years, many reports indicated that the rate of SPA has been increasing in China, India, Pakistan, Nepal, and neighbouring countries [Reference Fangtham and Wilde4–Reference Sur10]. Multiple antibiotic-resistant strains intensified the problem of treatment [Reference Fangtham and Wilde4, Reference Ochiai9, Reference Harish11]. The Chinese National Notifiable Disease surveillance system, established in the 1950s, showed consistently that the highest incidence of Salmonella infections occurred in the southwestern region of China, e.g. Guangxi Zhuang Autonomous (Guangxi); SPA accounted for about 10–40% of the total cases [Reference Fangtham and Wilde4, Reference Ochiai9, Reference Yang12].
No licensed vaccine is available for non-typhoidal salmonellosis since the removal of the whole cell parenteral TAB vaccine. In 1995, an efficacy trial of locally produced Vi typhoid vaccine in Guangxi demonstrated an efficacy of 70% [Reference Yang13]. Based on this and other clinical trials, Vi was licensed in China and mass immunization was introduced to Guangxi, particularly in school-aged children, with a consequent decline in the incidence of typhoid fever [Reference Acosta14]. In contrast, the number of SPA infections continued to rise and in Guilin region SPA had become the most common serogroup of Salmonella since the late 1990s [Reference Ochiai9, Reference Yang12]. This prompted the development of a new generation of SPA vaccines, one of which was the O-specific polysaccharide conjugate that was shown to be safe and immunogenic in clinical trials [Reference Robbins, Schneerson and Szu15–Reference Konadu17].
SPA, similar to S. Typhi, can be considered as a clone and is a pathogen for humans only with no animal reservoir [Reference Fangtham and Wilde4, Reference Holt18]. Thus, unlike other serotypes of Salmonella, the disease is transmitted through ingestion of faecally contaminated food or drinks from infected persons [19, Reference Walford and Noah20]. In the case of S. Typhi, chronic carriers are notoriously known to cause outbreaks, but the role of carriers in paratyphoid infections is not well established. SPA infections in Guangxi occurred mainly as outbreaks in institutions. Between 2000 and 2004, there were more than a dozen SPA outbreaks in middle and high schools where students lived in dormitories and had their meals at school canteens [Reference Yang12]. The sources of these outbreaks were sparsely investigated and rarely identified (authors' unpublished data).
Here, we review a large outbreak in November 2004 with the emphasis on serological carrier identification. Patient treatment, outbreak intervention measures, source investigation, carrier treatment and its implication on institutional outbreaks are discussed.
METHODS
Demographic background
Lingtian Middle School is located in the township of Lingtien, Lingchuan County, Guilin region, Guangxi, in the southwestern part of China. The population of Lingchung County is about 350 000. Most students are from farming families within ~10 km radius. The autumn term started on 28 August 2004 with 394 new students entering the 7th grade. The school had a total of 1005 students, 77 teachers and staff, and 10 school kitchen workers and all were included in the study. There were 20 classes from grades 7 to 9; the average class size was 50. The mean ages of students were (±s.d.) 13±0·40, 14±0·64 and 15±0·41 years for 7th, 8th and 9th grades, respectively. Because of the long distance between homes and school and the lack of transportation, 99% of the students lived in school dormitories during the academic year; about 15–20 students per room, each furnished with two long wooden sleeping platforms.
The town had no municipal water supply. The most common and also traditional method to treat drinking water was by boiling. The school water supply was pumped from a dedicated, covered concrete well, 6 m deep, near a creek and located 20 m outside the school fence. Water was charcoal-filtered without further treatment before being piped into the school for washing and cooking. Ten of the 20 classes had student-financed electric water boiling tanks in their classrooms used for the drinking-water supply. Supplemental boiled drinking water was provided by the central kitchen to all the classrooms, dormitories and drinking stations in the hall ways. Between 9 November and 14 November, heavy precipitation caused flooding in the nearby regions. The water-boiling tanks in the classrooms were frequently interrupted due to power shortages. Daily high temperatures during November ranged from 17°C to 23°C.
The students, kitchen workers and some staff had their meals regularly in school dining halls. All food was prepared by workers in the central school kitchen. For hygienic reasons, the kitchen did not serve cold cuts, salad or uncooked vegetables. Noodle, rice porridge, rice and soup were among the most common items served.
The school clinic has one physician and several part-time volunteers. The school is 16 km from Lingchuan County Hospital and the County Centre of Disease Control and Prevention (CDC), both institutions have microbiological laboratories. In immunization records of Lingchuan County CDC indicated that 92·5% of students were vaccinated with Vi typhoid vaccine between 1996 and 2002.
Case definition
A suspected case was defined as a person at Lingtian Middle School having one of the following clinical symptoms during 23 November to 7 December: fever (oral temperature ⩾37·7°C, duration ⩾24 h), diarrhoea, headache, sore throat, general malaise, anorexia, coughing, abdominal pain, or chill and with positive Widal test or with a positive blood culture. According to the Chinese Epidemiology Guideline 1996 those with a positive Widal test and having fever >3 days were identified as confirmed SPA infections. However, due to the large number of cases that occurred during the outbreak and the shortage of health staff, the collection of blood samples and clinical symptoms on days 2 and 3 was only undertaken when possible.
Microbiological investigation
Standardized Widal test (Salmonella O and H Serotyping kits, Lanzhou Institute of Biological Products, China, and Salmonella O antiserum factor 2, Difco, USA) was used for routine Salmonella screening at Lingchaun County CDC or at the County Hospital.
Blood culture was taken from patients with fever ⩾38·5°C whenever possible and from all the kitchen workers [Reference Konadu17, 19]. Briefly 1 ml blood was delivered into 9 ml enriched broth medium (glucose bile salt broth, Lanzhou Biological Products) incubated at 37°C for 1–7 days and checked visually each day for growth. When growth was suspected, the culture fluid was subcultured on MacConkey and Salmonella-Shigella agar plates (Shanghai Reagent Provision and Research Center for Diarrhoea Control, China). All initial identification and serotyping were performed at the microbiology laboratories in Lingchuan County Hospital or at Lingchuan County CDC, confirmed by Guangxi Regional CDC. Twelve isolates were sent to the Clinical Microbiology Laboratory, Clinical Centre, National Institutes of Health (NIH), USA for verification.
A rectal swab was taken from 44 patients and all kitchen workers during the investigation period; specimens were cultured on MacConkey and Salmonella-Shigella agar plates for isolation of SPA (see above). Repeated swab cultures were taken from 2 days to 28 days after the first swab depending on each case.
To rule out possible infection with other organisms, throat culture for influenza virus types A and B were performed on the three patients with a sore throat; blood samples from 17 patients were tested for IgM antibodies to influenza virus types A and B on 1 December 2004.
Outbreak source investigation
Samples of drinking water (n=6, 200–500 ml each) and food (n=5) were collected during or after the peak of the outbreak whenever possible and were cultured for SPA. A self-completion survey questionnaire of eating and drinking habits of the students was conducted by the Guilin City CDC after the outbreak.
Serum IgG anti-lipopolysaccharide (LPS) measurement
Serum IgG anti-LPS levels of 12 convalescents and of an equal number of age-matched healthy individuals in the same region were measured by ELISA. The assay was conducted at the National Institute of Child Health and Human Development, NIH, USA. Briefly LPS (1 μg/well), purified from SPA, was coated onto 96-well microtitre plates, serum samples were diluted in phosphate-buffered saline and plates blocked with 1% BSA. A serum sample (SPA101) from an earlier clinical trial of SPA conjugate vaccine was used as a reference and assigned 100 ELISA units (EU) [Reference Konadu16, Reference Konadu17].
Criteria for SPA carriage
We adopted the serological and microbiological methods for S. Typhi carriage identification [19]. A SPA carrier was defined as an individual from whom SPA was isolated in two positive swabs or stool cultures and had a serum anti-LPS IgG titre >200 EU. No further confirmation by gallbladder examination or sampling was performed in this study due to public health restrictions and limited capacities in the outbreak area.
Data analysis
The association of hygienic habits of students and the risk of SPA infection in suspected cases during the outbreak were compared in those with vs. those without the characteristics and statistically analysed by the χ2 test. Antibody levels are expressed as geometric mean (GM) EU and compared by two-sided t test.
RESULTS
Time-course
Figure 1 shows the time-course of the SPA infections in students attending Lingtien Middle School, before and during the outbreak. During the summer vacation there were sporadic cases in the communities: 17 clinically suspected and five blood culture-confirmed by Lingchuan County CDC. Shortly after the autumn term started, there was one large outbreak involving 95 suspected and six blood culture-confirmed cases. Reports of sporadic SPA infection at school continued throughout October and November.
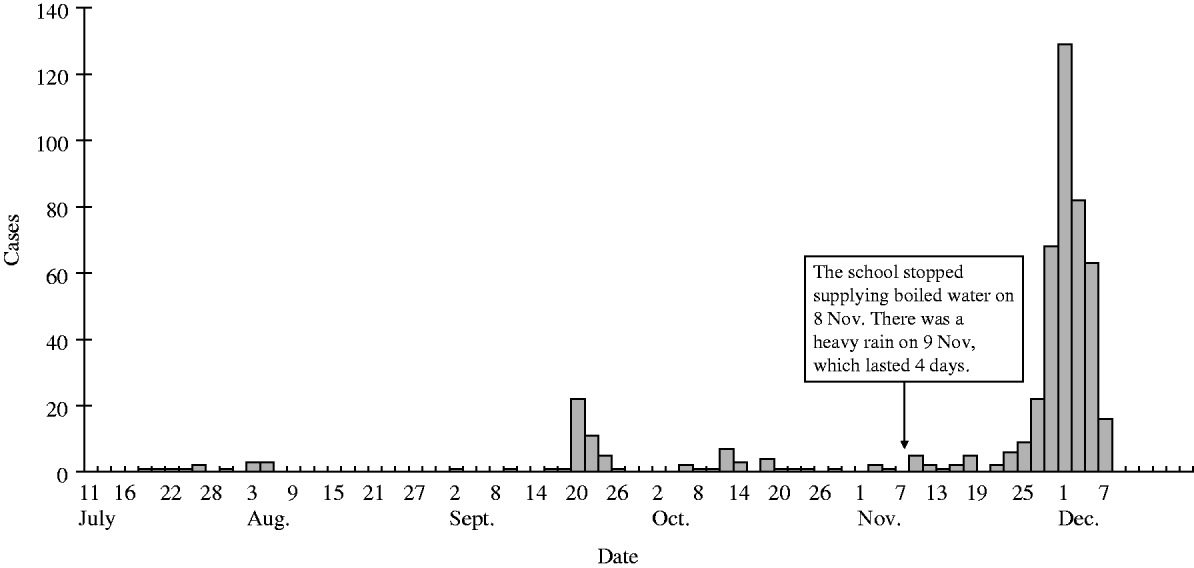
Fig. 1. Cases of Salmonella Paratyphi A in students attending Langtien Middle School by date of illness onset, from the beginning of the summer vacation (10 July 2004) to the end of the outbreak (7 December 2004). Cases during the summer (10 July to 30 August 2004) were from Lingchung CDC record.
On 23 November, three fever cases were reported in students from two separate classes. The first blood culture-positive SPA was identified by Lingchung County CDC on 27 November and confirmed by Guilin CDC 2 days later. Incidence steadily increased and peaked on 30 November. School-wide chemoprophylaxis with norfloxacin was started on 1 December. On 4 December, the epidemic control team from National CDC arrived on site and assembled a joint investigation team. The number of cases started to decline and there were none reported after 7 December. The outbreak lasted 14 days (23 November to 7 December) with 394 suspected cases (36·1% of total students and staff), of which 267 (67·8%) were identified as confirmed cases following the Chinese Epidemiology Guideline 1996.
Case distribution
The majority of the SPA cases were students (380, 96·5%), with a median age of 13·8 years. Table 1 shows the case distribution among students according to sex and grade. The incidence was highest in first-year students attending the 7th grade (220, 58%), followed by the 8th grade (89, 23%), and the 9th grade (71, 19%). The difference between the 7th grade and those in the 8th or 9th grades was significant (χ2=63·0, P<0·001). There were slightly more cases in male (203, 53%) than female (177, 47%, χ2=7·1, P<0·02) students. The case distribution in dormitory rooms ranged from 10% to 69% per room with the highest incidence in the rooms for lower-grade students (data not shown).
Table 1. Distribution of Salmonella Paratyphi A cases by profession, grade and sex
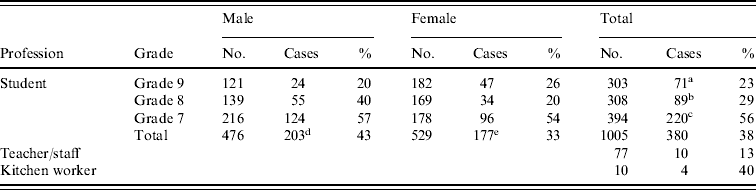
c vs. a, b (χ2>60, P<0·001); d vs. e (χ2=6·5; P<0·02).
Of the 10 kitchen workers there were four (40%) suspected cases. In contrast, there were only 10 (13%) in the 77 teachers and staff.
Symptoms
Table 2 shows the clinical symptoms of suspected cases. Fever was the most common symptom; more than 87% had a temperature >37·7°C (median 38·7°C; median duration 36 h). There were 62 patients (15%) with fever ⩾39°C. The second most common symptom was headache (59%), followed by fatigue (29%) and diarrhoea (28%). Twenty individuals were admitted to hospital (duration <2 days), where treatment consisted of oral antibiotics; only one patient was treated with intravenous antibiotics. There were no deaths associated with the outbreak.
Table 2. Clinical characteristics of patients in the Salmonella paratyphi A outbreakFootnote *
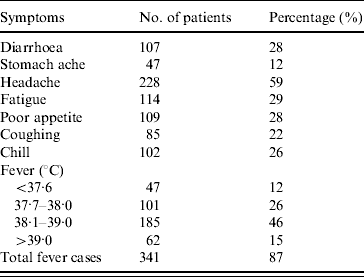
Case definition was: Widal test positive and having one of the clinical symptoms or having a temperature >37·7°C for >24 h.
* A total of 394 patients were included.
Microbiological findings
A total of 151 blood samples were cultured between 30 November and 2 December; 44 (29%) were positive for SPA. There were 54 rectal swabs collected during the outbreak, four (7%) were positive, of these, three were from kitchen workers (KW1, KW2, KW3). Two of these four also had positive blood cultures. A second rectal swab taken from a kitchen worker (KW1) 1 week after the first swab (9 December 2004) remained positive for SPA. Following intensive antibiotic treatment for 20 days, KW1's subsequent swab cultures taken on 23 and 28 December were negative. All tests for influenza virus of KW1 were negative. The remaining three people with positive swab cultures were not available for follow-up sample collection within the study time-frame.
All SPA isolates were confirmed by the Guilin County CDC and the Guangxi Autonomous CDC. Twelve samples from blood culture isolates were also verified by NIH, USA.
Treatment, prophylaxis and containment of the outbreak
In the past most SPA isolates from Guangxi region were sensitive to amoxicillin, ampicillin and norfloxiacin treatment. Therefore, starting on 30 November, all suspected patients were treated with ampicillin or amoxicillin (400 mg orally 3 times per day for 4 days; Southwest Pharmaceutical Company, Guilin). In an attempt to halt the sudden increase of SPA cases, chemoprophylaxis with norfloxacin (400 mg orally 3 times per day for 4 days) was provided on 1 December to the rest of the students and staff. For KW1 extended treatments with multiple antibiotics were given and weekly rectal swab culture performed whenever possible until SPA was not detected.
Student patients were assigned to an isolated dormitory until the outbreak was over. Kitchen workers with either positive swab or blood culture were excluded from kitchen duties.
Serological findings
The level of serum GM IgG anti-LPS in 12 convalescents, 6 weeks after the infection, was statistically higher than those of 12 healthy individuals (35·25 EU vs. 5·20 EU, P<0·001) (Table 3). One kitchen worker (KW1), had a significantly higher level of IgG anti-LPS (529·65 EU) than the GM of the convalescent sera.
Table 3. Serum IgG anti-LPS of Salmonella Paratyphi A in convalescents (6 weeks after infection), a chronic carrier, and in healthy students and adults
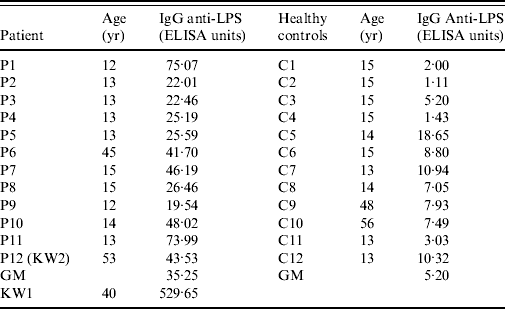
KW, Kitchen worker; GM, geometric mean.
IgG anti-LPS: patients vs. controls (35·25 vs. 5·20, P<0·001).
In August 2005, 8 months after the outbreak, public health officials collected additional blood samples from the three kitchen workers who had positive swabs during the outbreak and their anti-LPS titres were: 123·47 EU, 9·59EU and 6·22 EU for KW1, KW2 and KW3, respectively.
Identification and treatment of an SPA carrier
The three kitchen workers with positive swab culture were listed as suspected carriers. Only KW1 had serological support as a carrier. KW1 had a fever of 38·3°C, sore throat, acute upper respiratory symptoms, negative blood culture, positive Widal test, and positive rectal swab culture for SPA. She was diagnosed as having acute upper respiratory infection by the attending physicians and excluded as an SPA patient. Her second swab culture on 9 December remained positive. These characteristics, in part, fit the description of a carrier for Salmonella and KW1 was considered as an asymptomatic chronic SPA carrier.
KW1 was originally treated with cefotaxime (1·0-g i.v. drip twice a day for 6 days between 3 and 8 December) for respiratory infection. Subsequently her treatment was switched to a series of different antibiotics: ampicillin (1·0 g twice a day for 4 days, 9–12 December), norfloxacin (0·5 g twice a day for 7 days, 13–19 December), and finally SMZ–TMP (1·0 g twice a day for 2 weeks, 20 December to 2 January 2005). Rectal swabs collected from KW1 on 23 and 28 December were both negative for SPA.
Outbreak source investigations
There was no clear indication of a single source responsible for the outbreak. Food and water samples served immediately before and during the early period of the outbreak were not available for analysis. Between 2 December and 7 December, five food samples and six water samples (200–500 ml each) were collected from the water well, the creek next to the well, kitchen faucet, and hallway drinking stations, none were positive for SPA. During the outbreak, SPA cases were limited to people within the campus and no cases were reported from nearby communities, thus excluding flood-induced environmental contamination as a risk factor.
A form survey for potential risk factors was conducted after the outbreak, 146/162 (90%) questionnaires were returned. The results showed that, among individual hygienic habits, drinking unboiled water (χ2=15·0, P=0·002) and hand-washing without soap (χ2=5·3, P=0·02) were most strongly associated with contracting SPA infection (Table 4).
Table 4. Retrospective survey of hygiene habits in students and bivariate analysis of risk factors exposures
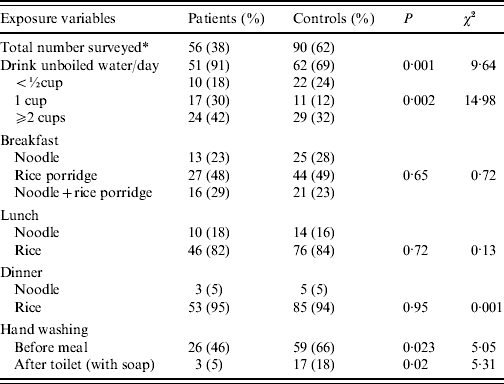
* 162 students surveyed, 146 (90%) returned the questionnaire. Of the returned forms: 56 patients, 90 controls; 68 male, 78 female, respectively. Patients were suspected SPA cases.
Because the majority of the positive swab cultures were from kitchen workers (75%) and at least one kitchen worker (KW1) was serologically consistent with carrier state, we speculate that faecally contaminated food or drink by one or more kitchen workers was one of the possible sources.
DISCUSSION
Salmonella Paratyphi A is a common cause of enteric fever in South East Asia. In Guangxi, outbreaks of Salmonella infections occurred mostly in middle or high schools and during the autumn term when students returned from summer vacation. One outbreak of S. Typhi that occurred in another high school of the same region was interrupted by Vi typhoid vaccination [Reference Yang21]. The SPA outbreak at Lingtien Middle School was one of the largest caused by Salmonella in the area. A total of 394 suspected cases were found during the outbreak and nearly all were students (96%). The incidence was significantly higher in new students entering the 7th grade (58%) than those in 8th and 9th grades. This trend has also been observed in other institutionally acquired diseases, such as meningococcal infections in college students and military recruits where the new entrants are usually more susceptible and had the highest risk of contracting the disease [Reference Yang21–Reference Harrison24]. The main reasons for this apparent clustering could be because first-year students, coming from diverse geographic and family backgrounds, never lived or dined in a communal setting, had little exposure to SPA and, unlike their senior schoolmates, were immunologically naive to the pathogen.
A total of 151 blood samples were screened, of which 44 were confirmed positive (29%). The sensitivity of blood culture for SPA is not well known [Reference Wang25], but based on the experience with S. Typhi, the volume of blood taken in this investigation may not have been sufficient [Reference Lanh26].
Unlike in the case of S. Typhi where disease transmission through carriers is well documented as a major cause of outbreaks, the carriage state of non-typhoidal Salmonella and its impact have not been characterized extensively [Reference Franklin27–Reference Anderson31]. In one report, a S. Paratyphi B outbreak in a restaurant setting, the source was traced to several family members excreting the organism intermittently [Reference Francis32]. In another report, chronic carriage of S. Paratyphi (subsp. not specified) was mentioned as a potential precursor of gallbladder cancer in comparison to S. Typhi carriage [Reference Caygill33]. SPA was isolated from the gallbladder of cholecystectomy patients: one recent report showed that 9/404 cases (carriage rate 2%) had SPA in the bile extracted, similar to that of S. Typhi (12/404, 3%) [Reference Khatri34]. Extrapolating from what is known about the serology of the carrier state in S. Typhi, serological data in this outbreaks suggested that at least one of the kitchen workers was a possible SPA carrier. The current study is the first to identify a possible chronic SPA carrier during an outbreak.
Salmonella carriers have been reported to harbour the bacteria in their gallbladder for a prolonged period of time and their immune systems were stimulated continuously by the bacterial surface antigens such as Vi for S. Typhi and LPS for SPA and other non-typhoidal Salmonella [Reference Gupta30, Reference Khatri34–Reference Lanata36]. For S. Typhi Vi antibody has been evaluated as a screening tool for the carriage state. LPS of SPA is a highly conserved carbohydrate antigen and, as a result, the level of serum antibody to LPS in carriers could be significantly higher than that of the convalescents, and yielded a positive Widal test. Thus anti-LPS measurement could serve as a powerful screening tool for identifying SPA carriers without the more invasive gallbladder sampling. Common definition of chronic carriage state is either long period (about 1 year) shedding of SPA or isolation of SPA from gallbladder. However, in practice, public health authorities are obligated to treat any suspected carriers until obtaining two subsequent negative stool cultures; this removes the possibility of prolonged carriage observation. Moreover, taking samples from gallbladder or stomach for SPA culture is too invasive to be accepted as a common practice. More examples correlating high anti-LPS titres with prolonged shedding of SPA in stool are needed to confirm this finding.
The source investigation in our study did not find SPA in water supplies. However, the form survey showed a strong correlation between drinking unboiled water with contracting the disease, but not with foodstuffs, indicating contaminated water was the most likely source of the outbreak. Several preset conditions such as heavy precipitation and frequent power shortages also supported this assumption. Another possible source of outbreak, in a closed institutional setting like this, could be attributed to contamination from food handlers who were either chronic carriers or had acute infections [Reference Todd37, Reference Torin38]. In the students' habit survey data, hand washing was an important factor for SPA infection, consistent with other published reports of the health benefits of proper hand hygiene [Reference Todd37]. Improper hand washing and bare hand contact with food are the most common misconducts in food preparation [Reference Pether and Gilbert39]. Bacteria can multiply in food and drinks to reach an infective dose, especially under warm ambient temperatures, as in the situation described here [Reference Fangtham and Wilde4, 19]. Other factors that worsened the initial contamination could be insufficient cooking temperature, long duration between preparation and serving, inadequate storage and cooling facilities, and lack of clean water.
This hypothesis has limitations. First, no food or drink samples prepared by the kitchen staff were proven to be contaminated or associated with illness in the student survey. Furthermore, there was no molecular or genetic epidemiological tracking to validate the linkage between the carrier strain and the outbreak isolates [Reference Ikumapayi40]. Unique to both subspecies, studies of genomic sequence of S. Typhi and SPA showed that each can be classified as evolving from a single source several thousand years ago [Reference Holt18, Reference Wain41]. This clonal nature of SPA has been consistently reflected in the epidemiological findings and biochemical culture characteristics [Reference Holt18, Reference Wain41]. Genomic examination of SPA isolates could provide an unambiguous answer to the source and pathway of transmission of the outbreak by fine discrimination of the isolates.
Unlike the situations of meningococcal infections and typhoid fever, institutional outbreaks can be prevented by immunization; however, there is no vaccine available to prevent SPA outbreaks [Reference Yang21–Reference Kumar, Murray and Havlichek23]. Preventive measures such as treatment of water supply, reinforcement of students' hygienic habits and enhancement of health screening of kitchen staff could help to eliminate major risk factors causing SPA outbreaks.
ACKNOWLEDGEMENTS
We thank the Clinical Microbiology Laboratory, Clinical Center, NIH, USA for verification of SPA isolates, Steven Hunt and Lingyun Zhou for technical help in serological assays, and Rachel Schneerson and John B. Robbins for helpful discussion and review of the manuscript. The work was supported by the intramural research of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health, USA and by the Guangxi Center of Disease Control and Prevention at Nanning, Guangxi, China.
DECLARATION OF INTEREST
None.







