INTRODUCTION
Enteroviruses are a leading cause of viral (aseptic) meningitis, which is one of the most common diseases of the central nervous system (CNS) worldwide [Reference Pallansch, Roos, Knipe, Howley and Griffin1]. These viruses are classified in the genus Enterovirus in the family Picornaviridae. The human pathogenic viruses responsible for 70–80% of the clinical cases belong to group B enteroviruses – coxsackie B viruses and various echoviruses. Echovirus serotypes 6, 9, and 30 are most commonly associated with viral meningitis outbreaks.
In accordance with national legislation, which defines the structure and conditions for surveillance of communicable diseases in the Republic of Bulgaria, all cases of viral meningitis and meningoencephalitis are notifiable. The definition of a patient with probable viral meningitis implies the presence of manifested clinical symptoms of the disease and laboratory parameters such as: colourless and clear cerebrospinal fluid (CSF) with pleocytosis, mainly due to lymphocytes, normal/slightly elevated protein and glucose levels; absence of Gram-stained microorganisms and/or negative microbiological culture of CSF. The definition of a confirmed case of viral meningitis implies the identification of the causative agent, through virological assays, performed on clinical samples (CSF, stool, sera) taken from the patient, consisting of: direct isolation on cell culture; detection of viral antigen or viral RNA via immunologically based tests and molecular-based techniques; and the detection of high antibody levels against the isolated virus in the patient's sera with seroconversion. Presence of high levels of IgM or IgA is an additional marker of acute infection.
In May 2012 in Plovdiv and Stara Zagora regions (South Bulgaria) an increased incidence of aseptic meningitis was observed. The number of hospitalized patients with aseptic meningitis (mostly children and adolescents) in the University Hospital for Active Treatment ‘St. George’ in Plovdiv and in the Multi-Profiled Hospital for Active Treatment ‘Prof. St. Kirkovich’, Stara Zagora, significantly increased over the following 4 months (May–August 2012).
This study describes clinical and epidemiological characteristics of an aseptic meningitis outbreak. The virological investigations revealed that the outbreak was caused by echovirus 30 (E30). The molecular analysis of 12 Bulgarian E30 isolates demonstrated a close correlation with an E30 strain isolated during an outbreak of aseptic meningitis in Greece in 2012, providing evidence for the circulation of this particular enterovirus type between the two neighbouring countries and its potential for spread over a large geographical area.
MATERIALS AND METHODS
A total of 220 clinical samples (85 faecal samples, 90 CSF samples, 21 nasopharyngeal swabs, 24 sera samples) from 157 patients suffering from aseptic meningitis were collected and sent for viral investigation to the National Reference Laboratory of Enteroviruses of the National Centre for Infectious and Parasitic Diseases (NCIPD), Sofia, Bulgaria. A case of aseptic meningitis was defined as illness characterized by acute onset, meningeal irritation, CSF pleocytosis [white blood cell (WBC) count >10/l] and negative bacterial culture.
A combined approach for enterovirus detection consisting of viral isolation on RD cell line and nucleic acid detection test was applied for 196 samples collected from 142 aseptic meningitis patients. Viral isolation was performed according to WHO recommendations [2]. Standard microneutralization assay for serotype identification was also performed on all enterovirus isolates detected using enterovirus antibody pools (RIVM enterovirus serotyping pools, National Institute of Public Health and the Environment, The Netherlands).
For the molecular-based diagnostics, enterovirus RNA was extracted from 10% stool suspension or CSF (200 μl) by the spin-column method (PureLink Viral RNA/DNA kit, Invitrogen, USA) and subjected to reverse transcription (RT) using random primers (Invitrogen, USA) followed by real-time polymerase chain reaction (PCR) with primers targeting the 5′-UTR region of the enterovirus genome as described previously [Reference Corless3]. Samples with cycle threshold >35 were not considered positive.
In addition, 24 sera samples were collected from patients with aseptic meningitis of which nine were from patients with faecal and/or CSF specimens available but negative by virus isolation and RT/real-time PCR, and 15 sera with no other samples available from discharged patients. Sera in dilutions of 1:8 to 1:64 were tested against a reference E30 strain using microneutralization assay in RD cell culture [2]. The serum titre was calculated as the reciprocal of the highest dilution at which 50% of E30-specific cell culture degeneration was observed.
A patient with confirmed E30 infection was defined as a person having E30 virus isolation or viral RNA detected directly in stool or CSF or an elevated titre (>64) of anti-E30 specific antibodies found in the serum sample.
A region of the protein encoding the VP1 gene of the 12 Bulgarian E30 isolates was amplified by semi-nested RT–PCR and a fragment of 312 nucleotides (nt) from position 2586 to position 2897 nt (according to the sequence of the reference human E30 strain Bastianni) was sequenced to further characterize these strains [Reference Nix, Oberste and Pallansh4]. Each RT–PCR product was sequenced in both directions to resolve the possible presence of ambiguous nucleotides (Macrogene, Seoul, Korea). The sequences obtained were edited and aligned by BioEdit v. 7.1.11 and Clustal W (www.mbio.ncsu.edu/bioedit/page2.html). Nucleotide similarity between Bulgarian E30 isolates and other E30 viruses published in GenBank was defined using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic analysis was performed by the Kimura two-parameter model as a model of nucleotide substitution and the phylogenetic tree was constructed using the neighbour-joining method (MEGA v. 5.1 software package). The statistical significance of the phylogenetic tree was estimated by bootstrap analysis with 100 pseudoreplicate datasets and bootstrap <70% was not shown in the phylogenetic tree.
Acession numbers
The Bulgarian E30 sequences reported in the present study have been deposited in GenBank and assigned accession numbers KF751630–KF751641.
The accession numbers of the sequences used for the construction of the phylogenetic tree are as follow: strain Bastianni (AF162711), Frater (AF081341), Giles (AF081342), PR-17 (AF081343), GR/EVs/Alexandroupolis.GRC/01·12 (KC309436), GR/EVs/Alexandroupolis.GRC/30·12 (KC309431), UK/CSF-1867/2007 (FJ525929), UK/CSF-08-2592/2008 (HQ897651), FR/313072-05 (AM711032), FR/CF196006-06 (AM711084), FR/csf260076/France2007 (AM946163), FR/csf260092/France2007 (AM946162), PAN/E30/2008 (HM854945), PAN/E30/2008 (HM854946), TUN/S99-06.TUN2006 (JN177732), FR/CSF1529/P17/2000 (AJ417347), FR/Nantes-30-12-05 (FM210459), FR/CF51-96/1996 (AJ430698), FR/CF613-96/1996 (AJ430701), FR/CF282-97/1997 (AJ430702), FR/CF1489-97/1997 (AJ430706), FR/CF521-96/1996 (AM237322), FR/CSF1650/P21/2000 (AJ417348).
RESULTS
Clinical data
A total of 157 individuals clinically diagnosed with aseptic meningitis were included in the study. Of the patients with suspected enteroviral infection major clinical features were fever, fatigue, headache, and repeated vomiting and photophobia. Myalgia was observed in 11%. Most patients had fever with a temperature between 38°C and 39°C (median 38·4°C). The signs of meningeal irritation (including neck stiffness, positive Kernig's and Brudzinski's signs, strengthened tendon reflexes) were milder and present in less than half of the clinical cases. A mean duration of these neurological signs was 5·1 days (±1·5 days). A severe constant headache that persisted even after the temperature had normalized was documented as the only sign showing CNS involvement in some of the patients (22%). Several diarrhoeal episodes were reported in 5% of the cases. The CSF laboratory examination at the moment of hospital admission showed mild to moderate pleocytosis (100–350 WBC/l, average 255 WBC/l) in 50% of the cases and in 67% of the patients a mononuclear predominance was documented. Slightly elevated protein and glucose levels were significantly less common, as these were documented in <21·2% of the aseptic meningitis patients. The course of the enteroviral illness was mild in most patients and led to complete recovery without neurological sequelae in all of the infected individuals. The initial therapy included intravenous fluids, diuretics such as 10% mannitol for 3 days and empirical application of antibiotics for the period before the results of the microbiology analyses were available.
Viral investigation
Viral isolation was performed over a total of 196 clinical materials (faecal specimens, nasopharyngeal swabs, CSF samples) collected from 142 aseptic meningitis patients. Of these, 117 samples (59·7%) from 99 (69·7%) individuals tested positive for enterovirus isolation in cell culture and/or RT–PCR. All isolates (n = 91) were serotyped by enterovirus pools (RIVM, The Netherlands) and E30 was identified as the major aetiological pathogen in 98·9% (90/91) of the cases. The remaining single isolate was characterized as coxsackie B. Most of the viruses were isolated from faecal specimens (72·9%, 62/85) and to a less extent from CSF samples (32·3%, 29/90); the lowest level of isolation was observed in nasopharyngeal swabs (19·1%, 4/21).
Serological tests were performed on 24 sera samples collected from 15 aseptic meningitis patients with no other clinical samples available and nine from patients for which no enterovirus was detected by isolation in cell cultures or molecular assay in the accompanying clinical samples. However, in 18/24 (75·0%) sera analysed a high titre of antibodies against E30 (>64) was found, which was an indirect sign of a recent E30 infection. Four (16·7%) patients had lower antibody titres (between 16 and 32) and for two patients titres were ⩽8 (Fig. 1). Unfortunately, it was not possible to determine a seroconversion because of the absence of additional sera collected in the reconvalescent phase.
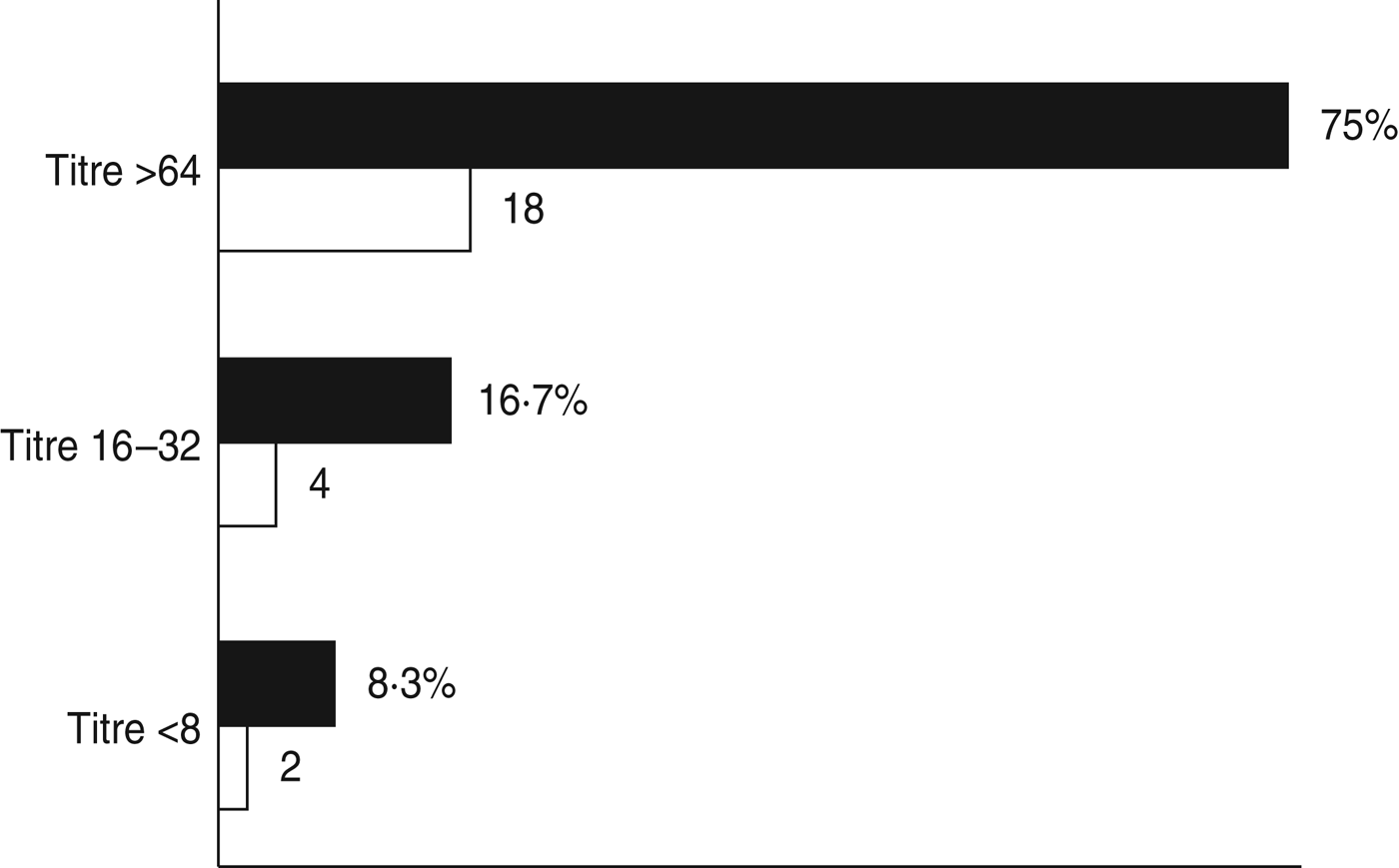
Fig. 1. Percentage (■) and number (□) of sera samples with antibodies against E30.
Sequencing of part of the VP1-encoding gene (the region between 2586 and 2897 nt) of 12 Bulgarian isolates revealed 99–100% homology between them at the nucleotide and amino-acid level. Moreover, 10 of the isolates were identical at their deduced amino-acid sequences, and only two strains BG/E30/483-PV/2012 and BG/E30/915-SZ/2012 had a single amino-acid variation. The alignment of the Bulgarian isolates with the E30 strains available in GenBank showed the closest relatedness (99–100% nucleotide and amino-acid similarity) with the Greek E30 strains isolated during an outbreak of aseptic meningitis that emerged in the Thrace region during the summer of 2012 [Reference Mantadakis5] (Fig. 2). Our E30 isolates also share nucleotide identities in the range 88–99% and amino-acid identity in the range of 98–99% with other E30 strains detected between 1996 and 2012 in France, UK, Tunisia and Panama [Reference Bailly6–Reference Martinez8], but are distantly related to the E30 reference strains Bastianni and Frater (14–19% nucleotide and 9–10% amino-acid divergence). In addition, the Bulgarian and Greek E30 isolates detected in 2012 differ in the region analysed in a single amino acid – Ile changed to Val (at position 2827 nt) with respect to the other E30 strains circulating during the previous years and reported in GenBank.

Fig. 2. Phylogenetic tree based on part of the VP1 genome segment (2586–2897 nt) of the 12 Bulgarian E30 strains investigated in this study and indicated by an asterisk (*). The tree was built with the neighbour-joining method using the Kimura two-parameter model as a model of nucleotide substitution.
Epidemiological data
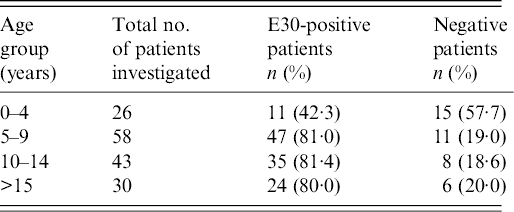
The 157 patients with aseptic meningitis analysed in the present study were aged between 5 months and 46 years (median age 10–11 years). The age distribution showed that the largest number of patients with E30-positive samples were in age groups 10–14 years (81·4%, 35/43) and 5–9 years (81·0%, 47/58), followed by adults aged >15 years (80·0%, 24/30) and children aged <4 years (42·3%, 11/26) (Table 1).
Table 1. Age distribution of the patients with E30-positive samples
The outbreak caused by E30 developed between May and August 2012 (weeks 20–35) with a peak at week 27 (Fig. 3). The first case with symptoms and isolation of E30 was detected in April (week 17) in a 2-year-old patient with aseptic meningitis resident in Plovdiv city. This was probably the index case of the outbreak from whom the virus began to spread sporadically first and then more extensively causing the epidemic.
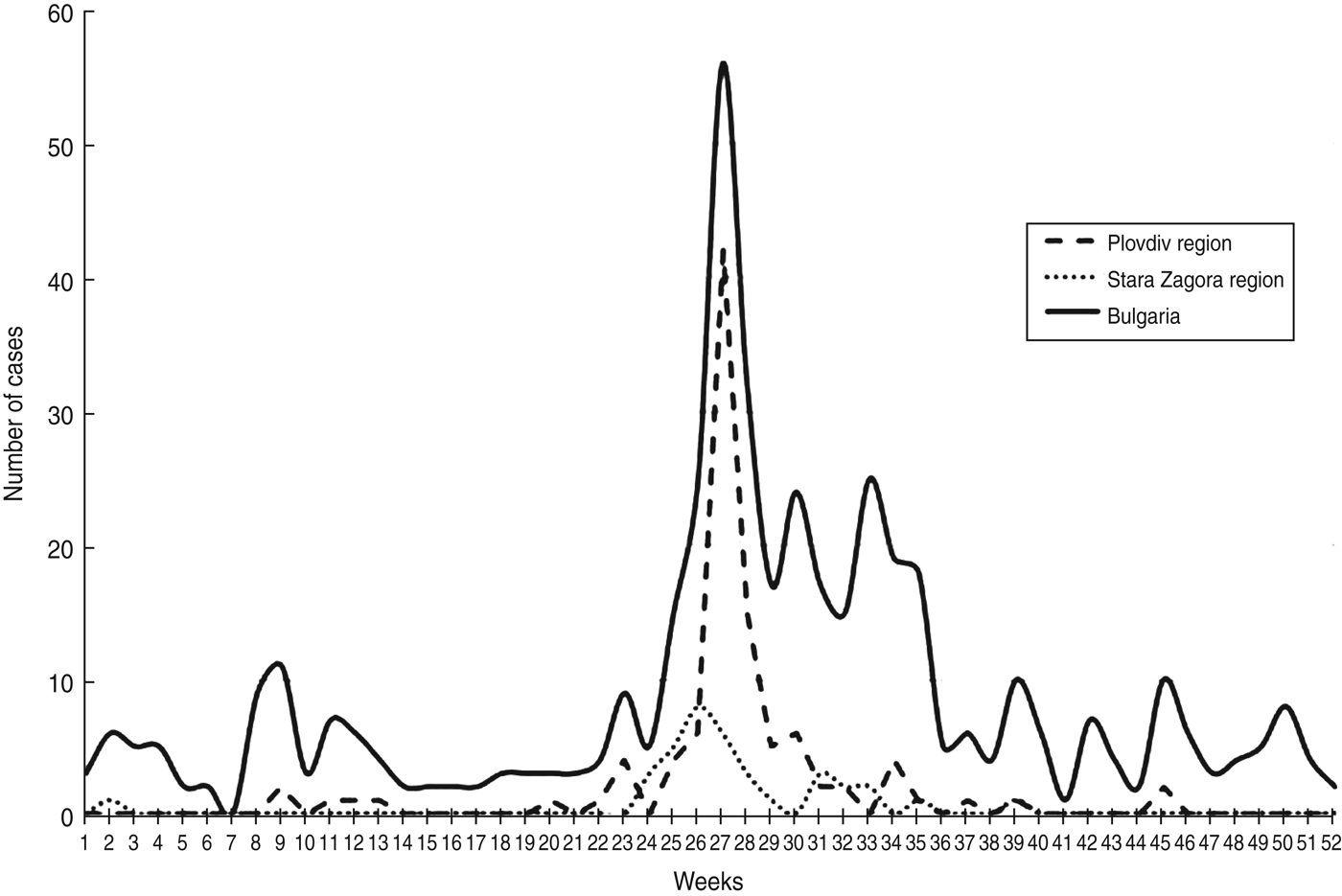
Fig. 3. Weekly distribution of the aseptic meningitis cases in Plovdiv region, Stara Zagora region and the whole of Bulgaria in 2012.
The vehicle of infection through which the virus was widespread remains unclear. The epidemiological surveys conducted showed that ten of the hospitalized patients frequented the same swimming pool (often described as a vehicle of contagious virus transmission via the fecal–oral route), and 27 had close contact with individuals showing clinical symptoms.
DISCUSSION
The outbreak of aseptic meningitis that occurred during the spring/summer of 2012 in the Plovdiv and Stara Zagora regions was due to an enterovirus infection. E30 was identified as the causative pathogen in 74·5% (117/157) of the cases using virological or serological methods, and therefore the outbreak was officially reported to be related to this particular echovirus serotype. Similar outbreaks caused by E30 are not rare and have been reported in many countries, e.g. Turkey, Romania, Switzerland, Brazil, China, Korea and others during the last two decades [Reference Ozkaya9–Reference Kim14].
The last outbreak caused by E30 occurred in Bulgaria in 2001 when the incidence of aseptic meningitis reached levels of 8·38/100 000 people, and E30 was detected in 74% of the patients with CNS infection. The present outbreak occurred during the spring/summer season (weeks 20–35), similarly to the 2001 outbreak, which developed from the end of June to the end of September (weeks 26–39); this is in accord with what has been observed for the circulation of enteroviruses which generally increase during the summer/autumn period in countries with temperate climate. Although a limited number of patients reported frequenting the same swimming pool in Plovdiv city, the vehicle of the echovirus infection remains undetermined. However, the limited number of individuals that initially developed the disease and the gradual increase of infected individuals in the following 4 months, suggest a conventional person-to-person viral spread.
Enterovirus aseptic meningitis is among the most common neuroinfections. The disease affects individuals from all ages, but most often children and adolescents. The outbreak described in this study affected mostly the 5–14 years age group. This can be explained by a low or absent circulation of E30 in the last 10 years, which generated a non-immune population in children and adolescents who had no contact with the virus during the previous outbreak. The increased incidence of E30 infection in adults in the current outbreak is most probably connected to the fact that as family members – parents, brothers and sisters – they were closely related to the infected children aged 5–14 years, i.e. the most heavily affected age group.
Sequencing of the 12 Bulgarian isolates confirmed they belonged to the E30 genotype as they showed >78% nucleotide and >88% amino-acid similarity with the reference strains Bastianni, Frater, Giles and PR-17. The molecular analysis also revealed very high nucleotide and amino-acid identity (99–100%) in the Bulgarian E30 isolates. Moreover, identical strains presenting the same nucleotide mutations were isolated both in Plovdiv and Stara Zagora regions indicating a spread of a single E30 genotype in the two cities during the different months, favoured by the frequent movement of people between these two cities. Indeed, the close nucleotide relatedness between the Bulgarian and Greek E30 strains with an overall variation of <1% strongly suggests that the circulation and spread of the strain were most likely facilitated by the active travelling of the residents of these two neighbouring countries during spring/summer holidays and demonstrates the potential of this enterovirus to be easily transmitted to a larger geographical area. Thus, establishing an international enterovirus molecular surveillance system for viral (aseptic) meningitis is desirable as a preventive measure and for the better understanding of enterovirus transmission and evolution.
DECLARATION OF INTEREST
None.






