INTRODUCTION
Diarrhoeal disease is among the most common causes of morbidity and mortality in developing countries such as Bangladesh. In all age groups severe diarrhoea can lead to hospitalization, serious sequelae such as haemolytic uraemic syndrome, and in some cases death [Reference Amirlak and Amirlak1]. Although most diarrhoeal episodes are self-limiting, it would be ideal to be able to prevent diarrhoea, especially the more severe episodes which have a higher likelihood of progressing to serious complications. Some prevention strategies such as improved water and sanitation and basic hygiene practices do not require knowledge of diarrhoeal aetiology, but others such as vaccination would benefit greatly from a comprehensive understanding of the overall burden of pathogen-specific diarrhoeal disease [Reference Fischer Walker, Sack and Black2].
Fluid and electrolyte replacement by oral hydration or intravenous fluid therapy is the treatment of choice for diarrhoeal disease. However, antibiotic therapy is indicated in some circumstances [Reference Taneja3]. The progressive increase in antimicrobial resistance among enteric pathogens in developing countries is becoming a critical area of concern. The acute diarrhoeal diseases for which antimicrobial therapy is clearly effective include shigellosis, cholera, and campylobacteriosis. However, for campylobacteriosis, the diagnosis is usually too late for antimicrobial therapy to be effective [Reference Sack4]. Of the bacteria causing diarrhoeal disease, Salmonella spp. continue to be a major public health problem. Although most Salmonella infections are self-limiting, serious sequelae, including systemic infection and death, can occur [Reference Tauxe5, Reference Mead6]. Incidence rates and aetiological agents of acute childhood diarrhoeal disease differ between developing and developed countries [Reference Thapar and Sanderson7]. Access to current antimicrobial susceptibility data is of importance to clinicians and is of particular significance to physicians treating hospitalized patients [Reference Karlowsky8]. Knowledge about susceptibility patterns of bacteria in different geographical areas is necessary to control bacterial resistance [9].
The aim of our study was to detect diarrhoea-causing bacterial pathogens in stool samples and rectal swabs collected from diarrhoeal patients hospitalized at Dhaka Hospital, ICDDR,B, Dhaka and domiciliary patients of Dhaka city. The intention was to observe the trends in bacterial pathogens associated with diarrhoeal diseases along with current antimicrobial susceptibility patterns of the isolated bacterial pathogens over a 4-year period.
METHODS
From January 2005 to December 2008, a total of 56 132 stool samples and rectal swabs were received from hospitalized (in-patients) and domiciliary (out-patients) diarrhoeal patients at Clinical Laboratory Services of ICDDR,B; Dhaka, Bangladesh. There were 15 965, 13 278, 13 137 and 13 752 samples in 2005, 2006, 2007 and 2008, respectively. It was not possible to exclude repeat samples from the same patient during an episode of diarrhoeal illness. All these samples were tested for the presence of Shigella, Salmonella, Vibrio and Campylobacter (when requested) and antimicrobial susceptibility tests were also performed.
Of the stool samples and rectal swabs received, a total of 15 783 samples (2533, 5104, 2299 and 5847 samples in four consecutive years) were tested for the presence of Campylobacter spp.
Bacteriological isolation
Collected stool samples and rectal swabs were directly inoculated onto McConkey (MC) agar (Difco, BBL), Salmonella-Shigella (SS) agar (Difco, BBL), taurocholate tellurite gelatin agar (TTGA) and Brucella agar (Difco, BBL) supplemented with 5% sheep's blood and five antibiotics (amphotericin B, cephalothin, polymyxin B, trimethoprim, vancomycin) for the isolation of Salmonella, Shigella, Vibrio and Campylobacter spp. respectively. All the plates were incubated at 37°C for 18–24 h except for Brucella agar, which was incubated at 42°C in an anaerobic jar with a CampyGen pack (CN0025, Oxoid Ltd, UK) for 48 h. Along with direct streaking, each sample was enriched in selenite broth (Difco, BBL) and bile peptone broth at 37°C for 18–24 h to enhance the isolation of Salmonella spp. and Vibrio spp., respectively. The enrichment broth for Salmonella was subcultured onto SS agar and the enrichment broth for Vibrio was subcultured onto TTGA agar and incubated at 37°C for 18–24 h. Bacterial enteric pathogens were identified by colony characteristics, and by biochemical tests using conventional and API 20 biochemical profiles (bioMérieux, France) when necessary. Isolates were further confirmed serologically using commercially available specific antisera (Denka Seiken, Japan). Campylobacter spp. isolates were differentiated as C. jejuni and C. coli by the hippurate hydrolysis test.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by the disc diffusion method on Mueller–Hinton agar (Difco, BBL) following CLSI guidelines [10]. For Campylobacter spp. blood agar containing 5% sheep's blood was used. Susceptibility testing was performed for ampicillin (10 μg), ceftriaxone (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), cotrimoxazole (25 μg), erythromycin (15 μg), nalidixic acid (30 μg) and tetracycline (30 μg). Antibiotic discs were obtained from Oxoid, UK. For V. cholerae, interpretive criteria for the zones of inhibition produced by ciprofloxacin and erythromycin discs have not been developed. However, interpretation was based on CLSI criteria for Enterobacteriaceae and multi-laboratory study findings, respectively [Reference Faruque11]. Interpretation of antimicrobial susceptibility for Campylobacter spp. was done using CASFM guidelines [12]. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains.
Statistical analysis
Trends in isolation as well as antimicrobial susceptibilities of isolated diarrhoeal pathogens were determined using χ2 for trend in Epi Info version 6 software (CDC, USA). A P value ⩽0·05 was considered significant for all comparisons.
RESULTS
During the study period from January 2005 to December 2008, a total of 56 132 stool samples and rectal swabs were received from diarrhoeal patients who ranged in age from 1 day to 80 years with a mean age of 13 years. For these four study years, 27·7%, 29·3%, 22·7% and 23·1% of the tested samples were found to be culture positive (P<0·001) and overall 25·7% of all the received samples were culture positive. In 2005, the isolates numbered 4424, whereas in 2006, 2007 and 2008, there were 3855, 2977 and 3172 isolations, respectively. Table 1 shows the distribution of individual species. Overall, Vibrio spp. were the most predominant microorganisms found to be associated with diarrhoeal diseases in this region. Shigella spp. were the second most frequently isolated pathogens. Other frequently isolated pathogens included Aeromonas spp., Salmonella spp., and Plesiomonas shigelloides.
Table 1. Bacterial pathogens isolated from diarrhoeal patients in Bangladesh from January 2005 to December 2008
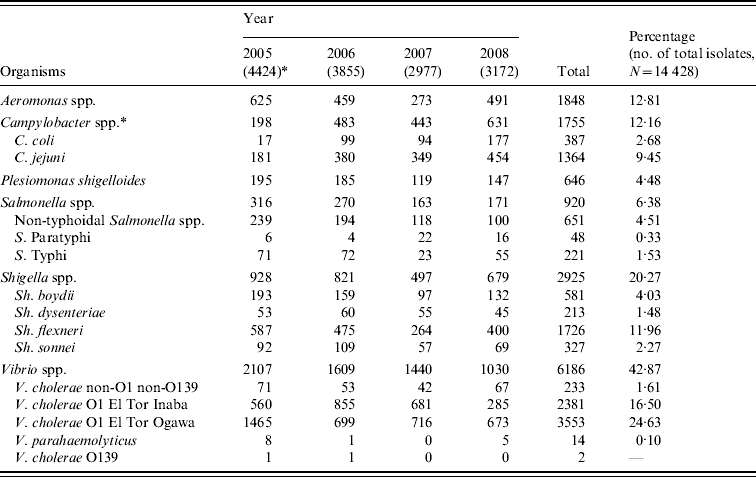
* Values in parentheses are the number of isolates in each year; Campylobacter spp. were isolated from 2533, 5104, 2299 and 5847 tested samples in four consecutive years.
Vibrio spp. isolation decreased to 32·5% of the total isolates in 2008, whereas in 2005, 2006 and 2007 they accounted for 47·6%, 41·7% and 48·4% of the total (χ2 for linear trend=110·6, P<0·001). Among the total Vibrio spp. isolated, 96% were identified as V. cholerae serogroup O1 El Tor biotype, 3·8% were V. cholerae serogroup non-O1 non-O139, and 0·2% were V. parahaemolyticus. V. cholerae O139 was identified only twice, once in 2005 and again in 2006. The V. cholerae O1 El Tor Ogawa serotype predominated throughout most of the study period although from August 2006 to August 2007 the Inaba serotype was more common (Fig. 1).
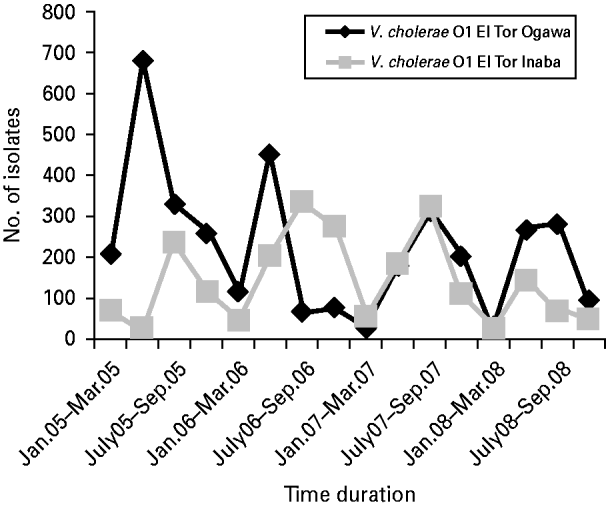
Fig. 1. Distribution of Vibrio cholerae O1 El Tor serotypes Ogawa and Inaba during the study period (January 2005 to December 2008).
A small decrease in the incidence of Shigella spp. was seen in 2007 but this was not statistically significant. Of the isolated Shigella spp., 59% were S. flexneri, 19·9% were S. boydii, 11·2% were S. sonnei and 7% were S. dysenteriae (not type 1). There was a decreasing trend in the isolation rate of Salmonella spp., they comprised 7·1%, 7·0%, 5·5% and 5·4% of the total isolates in four consecutive years (χ2 for linear trend=13·8, P<0·001). Non-typhoidal Salmonella spp. were more frequently isolated than typhoidal Salmonella spp. and there was also a decreasing trend in their isolation (χ2 for linear trend=25·4, P<0·001). There was a sharp increase in isolation of Campylobacter spp.; 7·8%, 9·5%, 19·3% and 10·8% of tested samples in four consecutive years revealed Campylobacter (χ2 for linear trend=25·1, P<0·001). During the study period, a total of 1755 Campylobacter isolates were obtained from the tested samples and of these isolates 77·7% were identified as C. jejuni and 22·1% as C. coli. In the first 3 years, there was a decreasing trend in isolation of Aeromonas spp. being 14·1%, 11·9% and 9·2% of the isolates (χ2 for linear trend=41·2, P<0·001); however, the isolation rate increased to 15·5% in 2008. There was no significant increase or decrease in the isolation of Plesiomonas shigelloides; overall they comprised 4·5% of the total isolates.
Overall, 99% of V. cholerae serogroup O1 isolates showed resistance to cotrimoxazole and 61% to tetracycline, but the isolates were susceptible to ciprofloxacin. Reduced susceptibility to erythromycin in serogroup O1 isolates increased significantly between 2005 and 2008 (P<0·001). Thirty-four percent of V. cholerae non-O1 non-O139 isolates showed resistance to cotrimoxazole, and reduced susceptibility to erythromycin increased from 7% in 2005 to 94% in 2008 (χ2 for linear trend=109·3, P<0·001). All the isolates were susceptible to ciprofloxacin (Table 2).
Table 2. Percentage of antimicrobial resistance in Vibrio cholerae O1 and non-O1 non-O139 isolates from diarrhoeal patients in Bangladesh
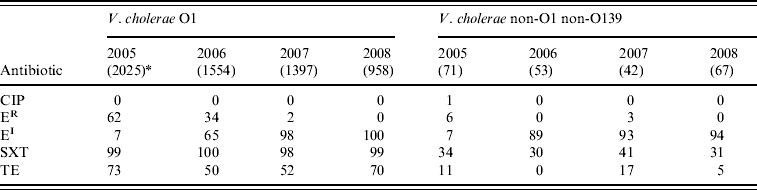
CIP, Ciprofloxacin; ER, erythromycin resistant; EI, erythromycin intermediate; SXT, cotrimoxazole; TE, tetracycline.
* Values in parentheses are the number of isolates.
Shigella spp. were increasingly resistant to nalidixic acid and ampicillin. Overall, 51%, 83% and 70% of the S. flexneri isolates showed resistance to ampicillin, nalidixic acid and cotrimoxazole, respectively and resistance to ciprofloxacin increased from 1% of isolates in 2005 to 34% in 2008 (χ2 for linear trend=262, P<0·001). Overall, 55%, 84% and 52% of S. boydii, S. sonnei and S. dysenteriae (not type 1), respectively showed resistance to nalidixic acid while cotrimoxazole resistance was 53%, 97%, and 73%, respectively. The overall resistance to ampicillin was below 40% for these isolates (Table 3). Overall, Salmonella spp. showed resistance to nalidixic acid (52%), ampicillin (30%), cotrimoxazole (24%) and chloramphenicol (19%); 33% showed reduced susceptibility to ciprofloxacin whereas 3% were completely resistant (Table 4).
Table 3. Percentage of antimicrobial resistance in Shigella spp. isolates from diarrhoeal patients in Bangladesh
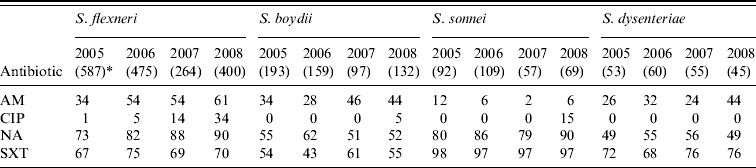
AM, Ampicillin; CIP, ciprofloxacin; NA, nalidixic acid; SXT, cotrimoxazole.
* Values in parentheses are the number of isolates.
Table 4. Percentage of antimicrobial resistance in Salmonella spp. isolates from diarrhoeal patients in Bangladesh
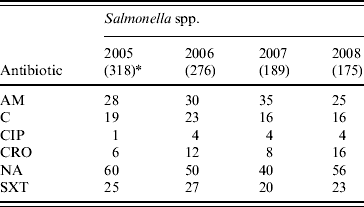
AM, Ampicillin; C, chloramphenicol; CIP, ciprofloxacin; CRO, ceftriaxone; NA, nalidixic acid; SXT, cotrimoxazole.
* Values in parentheses are the number of isolates.
Thirty-one percent and 37% of the Campylobacter isolates were resistant to ampicillin and tetracycline, while ciprofloxacin resistance increased from 65% in 2005 to 88% in 2008 (χ2 for linear trend=39·4, P<0·001). However, the isolates were mostly susceptible to erythromycin (Table 5).
Table 5. Percentage of antimicrobial resistance in Campylobacter spp. isolates from diarrhoeal patients in Bangladesh
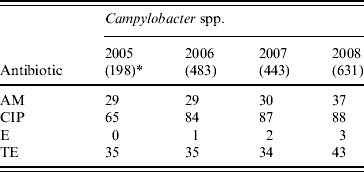
AM, Ampicillin; CIP, ciprofloxacin; E, erythromycin; TE, tetracycline.
* Values in parentheses are the number of isolates.
DISCUSSION
The present study traces the trends of bacterial pathogens associated with diarrhoeal disease in Dhaka, the capital city of Bangladesh, over a 4-year period. In addition, changes in the antimicrobial resistance patterns of the associated bacterial pathogens are presented.
V. cholerae O1, Shigella spp., enterotoxigenic Escherichia coli, C. jejuni and rotaviruses are important diarrhoeal pathogens in Bangladesh [Reference Qadri13–Reference Faruque19]. In the present study, prepotency of Vibrio spp. was observed in four consecutive years with a decreasing trend in isolation in the last year (2008). Among Vibrio spp., V. cholerae serogroup O1 El Tor biotype was the most predominant. Re-emergence of the Inaba serotype and a sharp decrease in isolation of Ogawa serotype from August 2006 to August 2007 is also indicated by our study. Shigella spp. prevailed as the second most isolated organism with a decreased isolation rate in 2007. S. flexneri predominated among the isolated Shigella spp. followed by S. boydii, S. sonnei and S. dysenteriae; however, no S. dysenteriae type 1 was isolated in the study period. This finding is similar to other previous reports from Bangladesh [Reference Bennish20] and other developing countries such as Brazil [Reference Lima21], Egypt [Reference Wasfy22], Indonesia [Reference Tjaniadi23], Tanzania [Reference Navia24] and Thailand [Reference Hoge25]. There was a decreasing trend in isolation of Salmonella spp.; however, for Campylobacter spp. the trend was increasing. In the first 3 years of the study Aeromonas spp. showed a decreasing trend in isolation but the isolation rate increased in 2008.
During the study period, except for Campylobacter spp., there was a decreasing trend in isolation of bacterial pathogens. Although the actual reason for this decreasing trend is not clear, it might be due to an increased awareness in the urban population of infection risks and consequent improvements in hygiene and sanitation practices. On the other hand, the increase in isolation of Campylobacter might be due to a change in food habits. In addition, improvements in laboratory techniques and staff practices might have had an indirect influence over the study period.
In the present study, V. cholerae isolates were frequently resistant to cotrimoxazole and tetracycline, but sensitive to ciprofloxacin; Shigella spp. showed varying degree of resistance to cotrimoxazole, nalidixic acid and ampicillin, and a sharp increase in ciprofloxacin resistance was also observed for S. flexneri isolates. Salmonella spp. showed resistance to nalidixic acid, ampicillin, cotrimoxazole and chloramphenicol, whereas a similar kind of study in Indonesia reported Shigella spp. increasingly resistant to ampicillin, cotrimoxazole, chloramphenicol and tetracycline. In an Indonesian study, Salmonella spp. were sensitive to all the antibiotics tested and a small number of V. cholerae O1 showed resistance to ampicillin, cotrimoxazole, chloramphenicol and tetracycline [Reference Tjaniadi23]. A slightly earlier report on two cholera outbreaks in Tanzania (1997 and 1999) showed a similarly high frequency of cotrimoxazole resistance in V. cholerae O1 isolates compared to the present study. Increasing resistance to chloramphenicol, ampicillin and tetracycline was also seen in the Tanzanian outbreaks [Reference Urassa26]. In another report Shigella spp. were found to be 81·8% resistant to ampicillin, 72·7% to chloramphenicol, 96·9% to tetracycline and 87·9% to cotrimoxazole in Tanzania [Reference Navia24]. In Bangladesh, multidrug resistance of V. cholerae O1 from urban and rural areas was reported, the strains were resistant to tetracycline, erythromycin cotrimoxazole and furazolidone; reversal of susceptibility to tetracycline of the strains after a 2-year period was also reported [Reference Faruque11].
Antibiotic resistance among the Salmonella spp. isolated was relatively frequent except for ciprofloxacin, where resistance was rare. In contrast, there was a marked increase in ciprofloxacin resistance in Campylobacter spp. between 2005 and 2008. An increase in ciprofloxacin-resistant Campylobacter strains has been reported worldwide with rates varying between 45% and 83% [Reference Luber27–Reference Senok29]. A systemic surveillance over an 11-year period in Karachi, Pakistan also reported a steady rise in resistance against ampicillin, tetracycline and ofloxacin in Campylobacter isolates [Reference Ibrahim, Zafar and Hasan30]. These findings call into question the use of ciprofloxacin as a drug of first choice for empirical treatment of campylobacteriosis. Campylobacter isolates resistant to erythromycin were quite rare and this antibiotic may be more useful for the treatment of campylobacteriosis in Dhaka.
The increase in antibiotic resistance observed in this study may be a reflection of the overuse and misuse of antibiotics due to their easy availability over the counter from local drug stores. Recent use of antibiotics in animal husbandry, and fruit and vegetable cultivation might have played some role in the transfer of resistance factors. Nosocomial infection by multidrug-resistant bacteria is common problem in Bangladesh [Reference Ryder31, Reference Darmstadt32], which is also an important cause of the emergence and spread of multidrug-resistant bacteria. To prevent the spread of antibiotic resistance among the diarrhoea-causing bacterial pathogens dispensing of antibiotics without a prescription should be restricted, community-wide education about the responsible use of antibiotics should be promoted, physicians should encourage patients to start antibiotic therapy after culture and sensitivity results have been obtained and patients should complete the full course of antibiotics.
Understanding the burden of pathogen-specific diarrhoeal disease is important for planning effective control programmes and for the overall reduction of diarrhoeal disease in persons of all ages. Current data on the local burden of bacterial pathogens and their susceptibility pattern will help physicians in the empirical treatment of diarrhoeal patients in this endemic area.
ACKNOWLEDGEMENTS
This study was funded by ICDDR,B and its donors who provided unrestricted support to ICDDR,B for its operations and research. Current donors providing unrestricted support include: Government of the People's Republic of Bangladesh; Canadian International Development Agency (CIDA), Embassy of the Kingdom of the Netherlands (EKN), Swedish International Development Cooperation Agency (Sida), and the Department for International Development, UK (DFID). We gratefully acknowledge these donors for their support and commitment to ICDDR,B's research efforts and activities.
DECLARATION OF INTEREST
None.








