INTRODUCTION
Brucellosis is one of the most common zoonotic infections worldwide and is a public health and economic burden in most developing countries [Reference Kracalik1]. Globally, more than 500 000 new cases of brucellosis are reported annually [Reference Asiimwe, Kansiime and Rwego2–Reference Abdullayev5]. However, the incidence rate of this disease varies from <0·01 to >200 cases/100 000 people, depending on the population [Reference Al-Anazi and Al-Jasser6], and the incidence is far higher in occupationally at-risk groups, such as agro-pastoral communities [Reference Al-Anazi and Al-Jasser6].
In Iran, around 16 000 cases of brucellosis are reported annually; this imposes a huge burden on the country [Reference Pappas7, Reference Pakzad8]. This burden is augmented by under-detection and underestimation of the disease due to inefficient surveillance systems for both humans and animals [Reference Al-Anazi and Al-Jasser6]. The migratory nomadic population numbers 1·2 million in Iran, most of whom endure poor living conditions; such as poor access to clean water. In addition, their pastoralist lifestyle entails regular contact with livestock, as they are involved in importing and exporting herds to other regions, and these individuals play an important role in producing, processing, and handling dairy products. In this study, we aimed to learn the prevalence and risk factors for brucellosis in this nomadic population in order to provide data for improved, evidence-based planning and interventions in the fight against this disease.
MATERIALS AND METHODS
General protocol
A 4-month (June–September 2015) cross-sectional serostudy was conducted to determine the prevalence of brucellosis in Iranian nomadic pastoralists. Eghlid, located in the north of the Fars province of Iran, is one of the most important nomadic poles and hosts a large seasonal migratory nomadic population of 10 000 people. This population lives in 2000 tents, occupying 19 regions that cover 7000 km2 while cohabitating with half a million livestock, mainly sheep and goats. Their pattern of migration takes them from warm areas in the south of Fars province to Eghlid city in spring, and eventually to hot areas in autumn. Each of the 19 regions in Eghlid maintains a healthcare centre that gives each nomadic family a unique code.
The required sample size for this study was calculated to be 578 participants, assuming an estimated 50% prevalence of brucellosis, a 95% confidence interval (CI), 5% error, and, due to the sampling method, a design effect of 1·5. To achieve a proportional-based systematic randomized sampling, we defined the sample size for each of the 19 regions based on their population size; we selected 18 individuals from the region with the smallest population up to 95 individuals from the region with the largest population. In order to find eligible individuals, families were first selected through a systematic randomized approach using the unique number that was given to each family by the regional healthcare centres. Then, one person from each family was selected by simple randomization and was interviewed in his/her tent.
Eleven teams were organized to conduct this study. Each team consisted of two endemic health sector staff members: an interviewer and a phlebotomist. The central laboratory of Eghlid city was also prepared to conduct laboratory tests according to the defined protocols. All teams were trained regarding the aims of this study, how to correctly complete the data collection forms, and regarding blood sampling and proper sample handling. The coded data collection form consisted of 31 questions designed to assess demographic characteristics, symptoms related to brucellosis (including fever, chills, sweating, headache, low back pain, arthralgia, myalgia), previous history of brucellosis or its treatment, number of livestock owned and their brucellosis vaccination status, history of animal abortion in the year prior to this study, participation of interviewees in the production, processing, and handling of dairy products, and the accessibility and use of personal protective equipment. The form also included the results of the following laboratory tests: the Rose Bengal plate test (RBPT), the serum agglutination test (SAT) test, the 2-mercaptoethanol (2-ME) test, and the Coombs Wright test (CWT). The forms were completed via face-to-face interviews in each tent after explaining the purpose of the research and obtaining consent. In the case of children, their mothers were interviewed. A 3-ml blood sample was taken from each participant and placed in blood tubes that were labelled with codes that matched the data collection forms. Blood samples were transported to the laboratory maintaining cold chain standards.
Laboratory protocol
A total of 536 blood samples were collected. The sera were separated by centrifuging the blood samples at 2000 g and kept at –20 °C until testing. The RBPT, SAT, 2-ME, and CWT were performed on all samples using whole-cell antigen produced by the Pasteur Institute for Health Sciences Research Company (Iran) based on their established protocol [Reference Zeinali, Shirzadi and Hajrasoliha9, Reference Hajia10]. For the RBPT, any degree of agglutination in bright light was interpreted as positive, and no agglutination was interpreted as negative [Reference Zeinali, Shirzadi and Hajrasoliha9]. The SAT was performed in eight sequential serum dilutions of 1:20, 1:40, 1:80, 1:160, 1:320, 1:640, 1:1280, and 1:2560, and the highest dilution with 50% agglutination was reported. An agglutination titre ⩾1:80 was considered positive [Reference Zeinali, Shirzadi and Hajrasoliha9, Reference Hajia10]. In the 2-ME test, eight sequential serum dilutions of 1:20 to 1:2560 were assessed, and titres ⩾1:40 were considered positive [Reference Zeinali, Shirzadi and Hajrasoliha9]. In the presence of SAT <1:80, CWT was considered positive if it yielded a 50% agglutination at a titre at least threefold higher than the SAT titre [Reference Zeinali, Shirzadi and Hajrasoliha9].
Diagnosis protocol
Taking into account the pastoral lifestyle of the nomad participants, symptomatic persons with positive SAT and positive 2-ME tests, or persons with a negative SAT and a positive CWT, were diagnosed with active brucellosis [Reference Zeinali, Shirzadi and Hajrasoliha9, Reference Andriopoulos11].
Data entry and analysis
The data from completed forms were extracted and entered into SPSS software v. 20 (IBM Corp., USA). The accuracy of data entry was ensured by checking completed forms against their corresponding data entered in the SPSS files. χ 2 and Mann–Whitney U tests were used for univariable analysis. The variables that met inclusion criteria for the regression model (P ⩽ 0·2) were entered into the logistic regression model. In the logistic regression (enter method), P values <0·05 were considered significant. Agreement ratios between serological tests were also calculated using a kappa formula.
Ethical statement
Strict ethical standards were maintained, including voluntary consent to participate, patient confidentiality of laboratory results, and standard, free-of-charge treatment for participants diagnosed with brucellosis. Furthermore, the research protocol of this study was approved by the Ethics Committee of the Health Policy Research Centre affiliated with Shiraz University of Medical Sciences. Finally, the authors assert that all procedures related to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
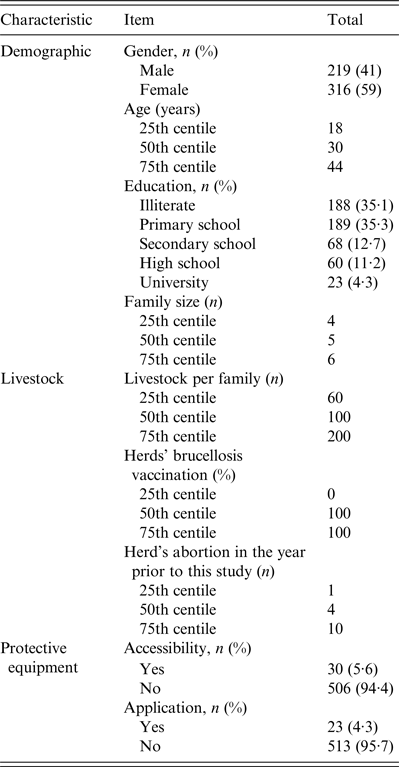
Of the 578 selected samples, 536 were included in this study, which reflects a 93% participation rate. The median age of interviewees was 30 (range 1–96) years. The female (316, 59%) to male (219, 41%) ratio was 1·4. The median family size of the participants was five and the median number of livestock per family was 100. A large proportion of the participants were illiterate (188, 35·1%) or reported education up to primary-school level (189, 35·3%) (Table 1). Of all participants, 449 (83·7%) reported involvement in animal grazing or dairy production, contact with aborted animal fetuses, or cleaning of corrals. In addition, 132 (24·6%) participants reported that their livestock had not been vaccinated against brucellosis. Furthermore, 282 (52·6%) participants reported a median abortion rate of four animals in their livestock during the year prior to this study. More than 506 (94·4%) responders denied owning or using protective equipment, such as masks and gloves, during animal contact (Table 1).
Table 1. Characteristics of the nomadic population in the seroepidemiological study of brucellosis in Fars province, Iran
Out of all participants, 325 (60·6%) reported clinical symptoms. In symptomatic persons, the RBPT was positive in 33 (6·1%) cases, the SAT was positive in 18 (3·3%) cases, and the 2-ME was positive in 30 (5·5%) cases. Moreover, 18 (3·3%) patients were positive according to both the SAT and 2-ME, and 36 (6·7%) patients were positive for both the SAT and CWT. In total, active brucellosis was detected in 54 participants, indicating a prevalence of 10% (95% CI 8–12). None of these patients had been previously diagnosed or treated for brucellosis. No significant association was noted between serological results and the presence or absence of clinical symptoms (Table 2).
Table 2. Results of brucellosis serological tests in the studied nomadic population of Fars province, Iran (n = 536)
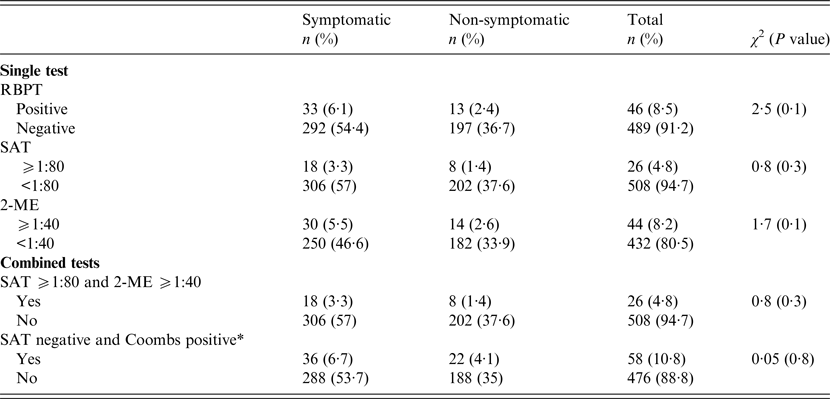
RBPT, Rose Bengal plate test; SAT, serum agglutination test; 2-ME, 2-mercaptoethanol agglutination test;
* Coombs test was considered positive by forming a 50% agglutination at a titre of at least threefold higher than the SAT titre <1:80.
Univariable analysis showed that the prevalence of brucellosis was associated with the age of patients (P = 0·01) and the number of abortions in the livestock (P = 0·01) (Table 3). According to logistic regression, however, there were no significant associations between brucellosis and these variables (Table 3). The kappa formula also demonstrated the following agreement ratios: RBPT/SAT: −67% (P < 0·001), RBPT/2-ME: −92% (P < 0·001), RBPT/active brucellosis: −29% (P < 0·001), and SAT/2-ME: −72% (P < 0·001). However, there was no significant agreement between CWT and each of RBPT, SAT or 2-ME tests (P ⩾ 0·1).
Table 3. Results of univariable analysis in the study of brucellosis of nomadic population in Fars province, Iran
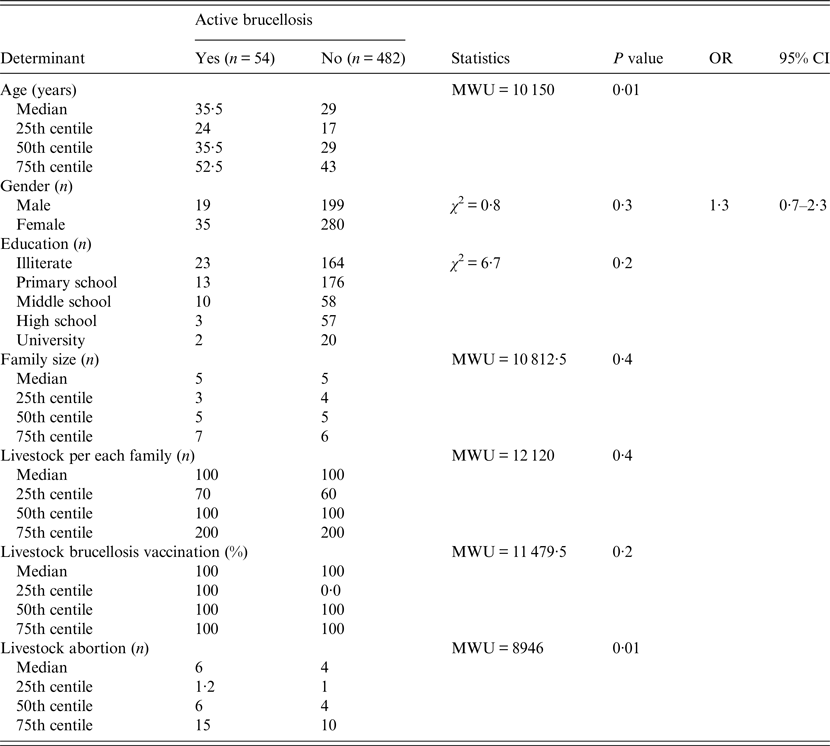
OR, Odds ratio; CI, confidence interval; MWU, Mann–Whitney U test.
DISCUSSION
Brucellosis has been eradicated from Norway (1952), Sweden (1957), Finland (1960), Denmark (1962), and England (1981) as well as The Netherlands, Austria, Japan, and Bulgaria (1985) [Reference Zeinali, Shirzadi and Hajrasoliha9]. However, this disease as a re-emergent zoonotic infectious disease is a major worldwide public health concern that is associated with a high economic burden [Reference Andriopoulos11, Reference Goksugur12]. Brucellosis has a very broad range of clinical manifestations from asymptomatic infection to a serious debilitating disease with diverse complications; therefore its diagnosis may be challenging [Reference Pappas7, Reference Goksugur12]. In Iran, brucellosis is an endemic disease, and despite improvement in its control, 16 000 cases of this disease are reported each year [Reference Maleki13–Reference Dean16].
The Iranian migratory nomadic population, known as ‘Ashaier’ people, comprise around 1 200 000 individuals (1·7% of the total population of Iran), most of whom practice a pastoralist lifestyle. This population manages >24 million sheep and goats, and produce 20% of the red meat and a significant proportion of all dairy products in Iran. Fars province, in the southwest of Iran, includes a large proportion (12% or 15 000 individuals) of this population, who collectively own 4 million livestock, mainly sheep and goats.
In this study, we found that 10% of this migratory nomadic population had active brucellosis, a rate that is far higher than the brucellosis prevalence (15·9/100 000) in the general population [Reference Zeinali, Shirzadi and Hajrasoliha9]. Their nomadic lifestyle and their close and continuous contact with livestock make brucellosis an endemic issue for this population that is challenging to diagnose [Reference Ari17]. Moreover, as this survey revealed, current interventions aimed at controlling brucellosis are insufficient due to delays in the detection of brucellosis patients via routine health system activities, a high abortion rate in livestock, low access and use of protective equipment, and incomplete brucellosis vaccination of livestock. In addition, we did not find any evidence that brucellosis vaccination of livestock in our region is effective. The health of this population is further complicated as a result of herds imported from neighbouring provinces with undefined brucellosis infection or brucellosis vaccination status, weaknesses in quarantine systems at provincial borders [Reference Esmaeili18], lack of herd insurance coverage, inadequate compensation for killed infected animals [Reference Esmaeili18], the practice of mixing animals who have recently experienced an abortion with healthy animals, drought in the settling areas of nomads, and low levels of education in the nomads [Reference Honarvar19].
There is some lack of consensus regarding the true incidence of brucellosis throughout Iran. One study conducted in rural areas of Isfahan, in central Iran, revealed that the incidence of brucellosis was 8·2/100 000 persons and that 15- to 20-year-old male subjects were more likely (2·1 times) to have this disease [Reference Zeinalian Dastjerdi, Fadaei Nobari and Ramazanpour14]. This finding contrasts with our results; the nomadic participants in our survey exhibited a very high prevalence of brucellosis without a significant gender difference. However, a separate study in the Kurdistan province, west of Iran, found a brucellosis incidence rate of 6–12% in the hunters, butchers, and healthcare workers of that area, which is similar to the findings in our study [Reference Esmaeili20]. In Khuzestan, southwest of Iran, the prevalence of brucellosis was found to be 6·3% in nomads, 1·5 times lower than the rate found in this investigation [Reference Alavi, Rafiei and Nikkhooi21]. On the other hand, a seroprevalence study of nomads in Lorestan province, west of Iran, found that 29·5% and 21·1% of nomads had positive SAT and 2-ME results, respectively [Reference Chegeni22]. In contrast, 3·3% of our participants tested positive according to the SAT and 5·5% tested positive according to the 2-ME test. Studies on this topic also disagree regarding the association between age and prevalence of brucellosis. While our study found no association between age and prevalence, the 10–19 years age group in the Lorestan study [Reference Chegeni22] and the 15–44 years age group in the Azna study [Reference Pakzad8] demonstrated a higher prevalence of brucellosis.
Studies in Azerbaijan and Uganda have proven the efficacy of herd immunization in controlling brucellosis [Reference Kracalik1, Reference Kansiime23]. Evidence also suggests that brucellosis control is not achievable unless at-risk groups are educated about this disease in both humans and animals; moreover, this education must involve a serious commitment on the part of both the public and private sectors [Reference Kansiime23, Reference Kansiime24]. Furthermore, studies indicate that health providers must look beyond index cases, as family members of brucellosis patients may also be in contact with common sources of infection and are therefore at risk of acquisition of this disease [Reference Sofian25–Reference Tabak27]; as such, familial screening in brucellosis cases may lead to early detection and prevention of brucellosis-related complications [Reference Pakzad8, Reference Sanodze26].
We found a two-thirds agreement ratio between RBPT and SAT and a one-third agreement ratio between RBPT and active brucellosis, indicating that brucellosis screening should not be based only on RBPT results. This finding is concurrent with that obtained in another study conducted in northeast Iran [Reference Sadeghian28]. The lack of a strong association between clinical symptoms and serological tests, as shown in this study, is supported by results from another study in which a series of patients underwent serological tests after confirmation of acute brucellosis [Reference Andriopoulos11].
Overall, our work indicates that routine health service activities cannot control brucellosis in the nomadic population in this region, and that a well-integrated surveillance system is needed. This finding is supported by evidence from other studies [Reference Pakzad8, Reference Leylabadlo, Bialvaei and Samadi Kafil15, Reference Dean16, Reference Joulaei29]. Annual active screening for brucellosis in nomadic populations is recommended before the establishment of a surveillance system. In addition, complete treatment of brucellosis patients, as well as screening for their family members, is an important component of a comprehensive brucellosis control programme. Additionally, we recommend implementing programmes to train nomads about this disease, including its transmission and prevention, as well as programmes that provide protective equipment to at-risk populations, support efforts for regular and complete brucellosis vaccination of livestock, and monitor brucellosis vaccination efficacy within herds. Additionally, one study [Reference Chegeni22] highlighted the importance of research-based field investigations in the fight against brucellosis. Finally, 5% of the screened population in our study demonstrated serological evidence of brucellosis despite being asymptomatic, and only 1/5 members of this group reported a previous history of treatment for brucellosis. Therefore, this group should be followed for possible development of clinical brucellosis.
Our study has a few limitations. Owing to the administrative gap between human and veterinary services in this area, we could not assess brucellosis in the livestock of brucellosis patients. Future studies that address this issue could provide more evidence regarding the risk factors for this disease in nomadic populations.
In conclusion, brucellosis is a common but neglected disease in the Iranian nomadic population. True brucellosis control will not be possible without a national commitment from all stakeholders, including communities as well as human and veterinary services.
ACKNOWLEDGEMENTS
We thank the nomadic populations for their honest participation as well as the staff of the health centres of Eghlid city, Fars province, Iran for assisting us in in performing this study.
DECLARATION OF INTEREST
None.






