INTRODUCTION
Foodborne diseases continue to represent a major public health issue worldwide [Reference Newell1]. In industrialized countries they occur primarily as self-limiting acute gastrointestinal illness (AGI), characterized by diarrhoea or vomiting. The impact of such diseases remains considerable on account of the high healthcare-related and social costs associated with their occurrence and sequelae. In addition, governments and the food industry are required to take measures to control foodborne pathogens along the food chain, since they represent a major cause of AGI even though AGI also includes episodes due to other routes of infection (waterborne and person-to-person contagion).
Estimating the frequency of AGI cases in a population is a central issue in evaluating the global burden of foodborne illnesses. Many of the agents involved in AGI are subject to surveillance in most industrialized countries. However, it is well known that data from passive routine surveillance programmes are frequently under-reported. To solve this problem, studies aimed at quantifying the true incidence of AGI have been developed, using information from symptom-based community surveys [Reference Wheeler2–Reference Majowicz4].
In Italy, surveillance of AGI is part of the official surveillance programme of infectious diseases (SIMI) and the laboratory-based surveillance network for enteric pathogens (Enter-net Italia).
AGI cases reported to SIMI fall into two categories: those associated with Salmonella spp. and those associated with other infectious agents. Cases reported to Enter-net are those from which bacterial enteric pathogens (Salmonella spp., Campylobacter spp., pathogenic Escherichia coli, Yersinia spp., Shigella spp., Vibrio spp.) have been isolated and typed.
Both systems are somewhat limited in terms of sensitivity, representativeness and completeness, and the information they provide is not really useful to estimate the burden of AGI in the general population.
Here we report the results of a retrospective survey aimed at estimating the occurrence and distribution of self-reported AGI in the Italian population. The protocol for the study was intended to give the highest level of comparability with similar studies and with parallel surveys conducted in other EU countries that, together with our study, were part of a larger project on prioritization of foodborne pathogens developed within the framework of the EU network of excellence on zoonoses MedVetNet.
METHODS
Study design
A retrospective nationwide sampling survey was conducted by telephone interview over a period of 12 months, between July 2008 and June 2009. The population under study comprised persons resident in Italy (n=59 619 290) in 2007 [5], with access to a private telephone landline. Based on figures provided by the Eurobarometer study [6] for 2009, 67% of Italian households had access to a private telephone landline.
A minimum of 3460 individuals was required to reach the target sample size. This was calculated based on a 10% expected monthly prevalence of AGI, 1% maximum allowable error and 95% level of confidence, using the formula for simple random samples outlined by Cochran [Reference Cochran7].
A two-stage sampling method was applied in order to randomly select respondents. First, the telephone number was randomly chosen from a roster of residential telephone landlines. The random selection of one person per household was then achieved using the next-birthday method [Reference O'Brien8]. The sample was stratified by age and geographical area of residence, based on demographic information on the resident population in 2007 [5].
Data collection
The target number of completed interviews was homogeneously distributed throughout the duration of the study period, in 12 monthly waves. The interviews were performed within the first 2 days of each month of the survey by an external company (CRA srl, Milan, Italy), using the computer-assisted telephone interviewing system (CATI). For each telephone line and for each household member selected, up to five attempts at contact were made, on different days and at different times. Unsuccessful contacts were categorized as follows: generic refusal, non-respondent households, replacement due to completed sampling quota, other (e.g. interrupted interviews).
Once oral consent to participate in the study was obtained, the respondent was requested to complete the interview using a standardized questionnaire (see Supplementary online material). In the case of participants aged <18 years, both consent and the answers to the interview were provided by one of the parents or the guardian, on behalf of the participant.
The questionnaire was developed from the core questions in the questionnaire used in the UK for the IID2 study on diarrhoeal disease [Reference O'Brien8] and adapted to the situation in Italy, especially with regard to organization of the healthcare system.
The interview was conducted in Italian. For each participant, beside general demographic and socioeconomic data, information was collected on the occurrence and severity of gastrointestinal symptoms such as diarrhoea, vomiting and other concurrent symptoms (nausea, abdominal pain, loss of appetite, fever, sneezing/coughing, sore throat, headache) which had manifested during the month prior to the date of the interview. The presence of conditions such as chronic diseases affecting the gastrointestinal tract or which had required, in the same period, surgical treatment of the stomach or bowel, were also recorded. Information on recourse to healthcare, laboratory tests, use of medication and the degree of disability associated with the occurrence of AGI was collected only for subjects who reported gastrointestinal symptoms during the recall period. All the data were obtained using closed-ended questions, with the exception of those concerning the names of medication/drugs, types of surgical treatment and chronic diseases, which were open-ended questions. The trained interviewers were given no options to elaborate on the questions.
Case definition and recall period
According to the International Collaboration on Enteric Disease ‘Burden of Illness’ [Reference Majowicz4], a case of AGI was defined as a person reporting three or more loose stools or any vomiting in a 24-h period that was not due to the consumption of drugs or alcohol, excluding those with cancer of the bowel, irritable bowel syndrome, Crohn's disease, ulcerative colitis, cystic fibrosis, coeliac disease or other chronic illnesses with symptoms of diarrhoea or vomiting.
Because there is evidence in the literature that gastroenteric symptoms may occur as a result of infections acquired primarily via the respiratory route [Reference Hall9], the use of such a syndrome-based definition of AGI could potentially lead to an overestimation of the burden AGI acquired via food or water. To take into account this possibility, the occurrence and distribution of AGI were estimated considering both all reported cases of AGI, to ensure comparability of the results with studies adopting the same standard case definition, or only cases without concurrent respiratory symptoms.
The period investigated was the 30 days prior to the interview. As the interviews were administered at the beginning of each month (first 2 days), we believed that using a recall period identical to the previous calendar month, rather than the more frequently used period of 28 days, could help respondents to identify more clearly the period of observation.
Analyses
Categorical variables were described using counts and percentages, and the relative 95% confidence interval (CI). Differences in proportion were assessed by the χ2 test or, when appropriate, Fisher's exact test. Mean value, median and range were used to describe continuous variables.
Demographic data for 2007 from the Italian National Institute of Statistics (ISTAT) were used to evaluate, by comparison, the representativeness of the sample [5].
Formulas for prevalence and incidence rate calculations are reported in the Appendix. The monthly prevalence of AGI was calculated as the proportion of survey respondents who reported episodes of AGI in the previous 30 days. The point prevalence of AGI was obtained as the proportion of cases with symptoms on the day of the interview. The annual incidence rate of AGI was adjusted, as outlined by Majowicz et al. [Reference Majowicz10], to account for cases of illness who developed AGI before the 30-days recall period and were still ill at the beginning of the observation period (pre-existing cases), under the assumption that cases occurred equally throughout the 30-day observation period.
Univariable and multivariable logistic regression analyses were applied to identify the factors associated with the occurrence of AGI. For this purpose, the characteristics of AGI cases were compared with those of respondents who reported no gastrointestinal symptoms or reported diarrhoea and/or vomiting that did not meet the criteria for classification as cases of AGI. The outcome variable was ‘being or not being a case of AGI’, according to the case definition adopted, while the characteristics and variables associated with respondents were the explanatory variables. Continuous variables, such as age, were recoded as categorical variables, prior to entering the analysis. Variables included in the final multivariable model were identified using the likelihood ratio test (P<0·05) to compare reduced and full models. To make sure that variables not included in the model were not confounders, these were added to the final model and tested for significance and confounding effect. This was assessed by looking for a change of ⩾30% in the model coefficients. Possible interactions between variables were assessed for statistical significance against the null hypothesis of no difference in the model coefficients with and without interaction terms, at the 0·05 alpha level, and only significant interaction terms were kept in the final model. Goodness-of-fit of the models was assessed using Pearson's χ2 test, with P⩾0·05 indicating an acceptable fit.
The same analytical approach was used to identify the factors associated with recourse to medical care. Of the AGI cases, the characteristics of patients who sought medical care were compared with those who did not. The outcome variable was ‘visited or not visited by a physician’ and the explanatory variables included socio-demographic characteristics, types of symptom, duration of illness and presence of concurrent symptoms. The same strategy used to identify factors associated with the occurrence of AGI was adopted for the multivariable model.
The statistical analyses were performed using SPSS v. 17.0 (SPSS Inc., USA).
RESULTS
Response rate and representativeness of the sample
To complete the 3490 telephone interviews, 8828 telephone contacts were required, yielding an overall response rate of 39·5%. A total of 5254 individuals refused to participate in the study, while for 84 consenting participants the interview could be administered only partially. The mean duration of the completed interviews was 3 min 38 s for respondents without symptoms, and 8 min 15 s for those reporting symptoms during the recall period.
The demographic features of the respondents were not statistically different from the official data on the general population (Table 1) except for age and citizenship, with subjects aged >75 years and immigrants being under-represented in the survey sample (P<0·01).
Table 1. Characteristics of respondents (n=3490), estimates of the monthly prevalence (95% CI) and annual incidence rate (95% CI) of self-reported acute gastrointestinal illness (AGI) in the 12-month national telephone survey performed in Italy between June 2008 and May 2009
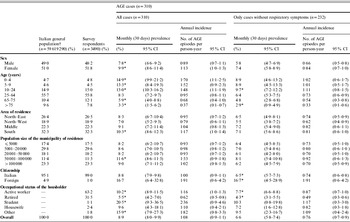
CI, Confidence interval.
† Data from the Italian National Institute of Statistics (ISTAT), 2007.
* Proportion per category significantly different (P<0·05) from all other categories combined tested by χ2 test.
Burden and distribution of AGI
The occurrence in the recall period of gastrointestinal symptoms such as vomiting and/or diarrhoea, consistent with the case definition criteria, was reported by 310 respondents. Seventy-eight of these reported concomitant respiratory symptoms such as coughing, sneezing, nasal discharge or sore throat. Overall, the monthly prevalence of self-reported AGI was 8·9% (95% CI 8·0–9·9) corresponding to an incidence rate of 1·08 AGI episodes/person-year (95% CI 0·90–1·14). Considering only cases of AGI without respiratory symptoms, the monthly prevalence was 6·6% (95% CI 5·8–7·4) and the annualized incidence rate 0·76 AGI episodes/person-year (95% CI 0·66–0·86).
Twenty-four respondents reported vomiting or diarrhoea on the date of the interview, yielding an AGI point prevalence of 0·69% (95% CI 0·42–0·98). Information on the day of onset of symptoms was available for 220 AGI cases.
Estimates of the monthly prevalence and incidence of AGI by demographic characteristics are reported in Table 1. Considering all AGI cases, the monthly prevalence was significantly higher in females than in males and, compared to the rest of the sample, in subjects aged <24 years. In particular, the highest and statistically significant differences between females and males were observed in the 10–24 years (P<0·01) and 25–64 years (P=0·03) age groups.
The prevalence of AGI was also higher in the South than in the rest of Italy, among immigrants than among Italians, and in households where the householder was a university student.
The effect of removing AGI cases with respiratory symptoms did not significantly modify the pattern of occurrence of illness within the population subgroups, except for the age and area-of-residence variables: a markedly lower monthly AGI prevalence was observed for persons aged <10 years and for residents in southern Italy.
A clear seasonal pattern of AGI emerged, with incidence rates peaking in late summer and in the winter months (November 2008 to March 2009) whether or not the cases with respiratory symptoms were excluded (Fig. 1). Seasonality was particularly evident for AGI in which diarrhoea alone or diarrhoea and vomiting were the primary symptoms, while cases of vomiting alone were distributed fairly homogenously over the study period. It is noteworthy that the seasonality pattern for children aged <5 years was quite different from that of the rest of the population, with marked peaks in the winter months and incidence rates lower than those observed in the other age groups during the rest of the year (Fig. 2).

Fig. 1. Incidence of self-reported acute gastroenteritis illness (AGI) per person-year, by study month and by primary symptoms in a 12-month national telephone survey performed in Italy between June 2008 and May 2009 (n=3490). (a) All AGI cases (n=310); (b) AGI cases without concurrent respiratory symptoms (n=232). * Three-month rolling average incidence.

Fig. 2. Three-month rolling average incidence of self-reported acute gastroenteritis illness (AGI) per person-year, by age group in a 12-month national telephone survey performed in Italy between June 2008 and May 2009 (n=3490). (a) All AGI cases (n=310); (b) AGI cases without concurrent respiratory symptoms (n=232).
Results of the univariable and multivariable logistic regression analyses to identify factors associated with the occurrence of AGI are reported in Table 2. The final model included only sex and age. No interaction between the variables emerged. The final model fitted the data well, as assessed by Pearson's χ2 test (P=0·23).
Table 2. Association of risk factors with the occurrence of self-reported acute gastrointestinal illness (AGI) in respondents (n=3490) included in a 12-month national telephone survey performed in Italy between June 2008 and May 2009: results of univariable and multivariable logistic regression analyses
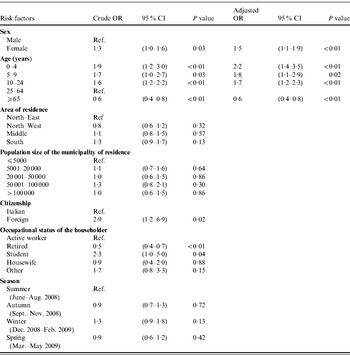
OR, Odds ratio; CI, confidence interval.
Severity of AGI and recourse to medical care
Of the 310 cases of AGI reported, 167 (53·9%) were marked by vomiting alone, 92 (29·7%) by diarrhoea alone, and 51 (16·5%) by both vomiting and diarrhoea. Bloody diarrhoea was reported in only one case. Information on the duration of illness, presence of concurrent symptoms and recourse to healthcare is reported in Table 3. Overall, the mean duration of illness was 3·22 days (range 1–30). The duration of illness was longer when both diarrhoea and vomiting were present than when diarrhoea or vomiting occurred alone. Although the difference was not significant, clinical conditions were more severe in the former cases, with a higher number of daily loose stools or vomiting on the peak day of illness, and the presence of other concurrent symptoms. Subjects who visited a physician and took medication accounted for a significantly higher proportion of respondents who reported diarrhoea than those who reported vomiting alone (P<0·01).
Table 3. Clinical features of illness (duration, number and type of concurrent symptoms) and recourse to medical care in cases (n=310) of self-reported acute gastrointestinal illness (AGI) observed in Italy between June 2008 and May 2009 in a 12-month national telephone survey
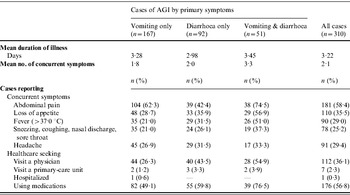
Overall, the number of respondents who sought medical assistance was 113 (36·5%). Of these, seven (2·3%) were requested to submit a stool specimen for diagnostic investigation and three (0·3%) respondents complied with the request. Hospitalization was reported by one subject, while medication was used by 176 (56·8%) subjects, on the advice or prescription of a physician (n=106) or pharmacist (n=22), or as self-medication (n=48). The most frequently used medications were probiotics (n=58), anti-motility and/or anti-emetic agents (n=43) and antibiotics (n=20). Details of recourse to healthcare and medication by socio-demographic variables are reported in Table 4, together with information on the restriction of daily routine activities and the loss of work/school days due to the occurrence of illness.
Table 4. Characteristics of cases (n=310) of acute gastrointestinal illness (AGI) and proportion of cases seeking medical care, using medications, reporting disability and loss of work/school days due to AGI in a 12-month national telephone survey performed in Italy between June 2008 and May 2009

* Proportion per category significantly different (P<0·05, χ2 test or Fisher's exact test) than all other categories combined.
Results of the univariable and multivariable logistic regression analyses to identify the factors associated with recourse to medical care are reported in Table 5. The variables finally included in the model were: age, duration of illness, type of primary symptoms, and presence of fever. No significant interaction terms between variables could be found at the P<0·05 level. Results of Pearson's χ2 test yielded a P value of 0·65, indicating that the model fitted the data well.
Table 5. Associations of risk factors with the recourse to medical care in cases (n=310) of self-reported acute gastrointestinal illness (AGI) identified in Italy in a 12-month national telephone survey, between June 2008 and May 2009: results of univariable and multivariable logistic regression analyses
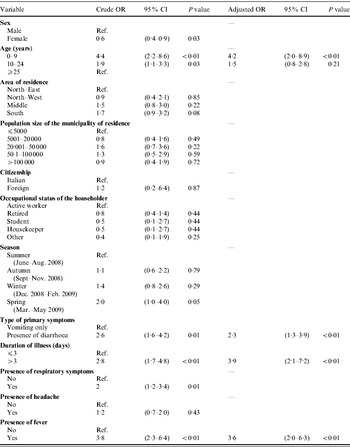
OR, Odds ratio; CI, confidence interval.
DISCUSSION
This is the first nationwide survey conducted in Italy with a view to describing the magnitude and distribution of AGI in the general population. Our results show that the burden of AGI in Italy is important. Based on the estimates of 1·08 self-reported AGI episodes/person-year and 36·0% of subjects seeking medical care for AGI, more than 4 million cases of illness and 1 million medical consultations occur each month throughout the country. When cases of AGI with concurrent respiratory symptoms were excluded from the analysis the annualized incidence rate dropped to 0·76 AGI episodes/person-year, a reduction of 29·6%. This variation is fairly similar to those reported by similar surveys conducted in the USA, Australia and Canada [Reference Hall9]. Interestingly, the largest variation in the incidence of AGI was observed in the youngest subjects who represent the population group with the highest demand for medical care and medication, and the highest impact in terms of indirect social costs due to the loss of work or school days of the affected subjects or their caregivers. Thus, the effect of including or excluding cases of illness with respiratory symptoms would markedly change the estimates of the global burden and costs associated with AGI. Understanding whether respiratory symptoms associated with AGI should be considered a proxy for AGI of non-foodborne origin is therefore a crucial issue, in our opinion, when estimating the burden of foodborne diseases.
The annual rate of AGI observed in Italy falls within the range of incidence reported in similar studies performed in other industrialized countries. When compared with parallel studies conducted in other EU countries using the same case definition [Reference Baumann-Popczyk11, Reference Müller12], and with the estimates from other studies adjusted for a different case definition [Reference Majowicz4], the incidence of AGI in Italy appears to be higher than those reported for Malta (0·4 episodes/person-year), Ireland (0·6), and the USA (0·8), similar to those reported for Poland (0·9), Canada (0·9) and Australia (1·0), and slightly lower than that reported for Denmark (1·4). The extremely low proportion of respondents who reported bloody diarrhoea and hospitalization (1/310 AGI cases, for both conditions), which was the lowest in similar surveys that adopted a similar case definition, suggests that our study was particularly sensitive in capturing mild cases of AGI. This could explain the rather high incidence observed in our study.
We have also to consider that our estimates may have been biased by several factors. Some inflation could possibly have occurred on account of ‘telescoping’, a recall bias leading respondents to attribute to the survey period episodes of AGI that occurred earlier, as described previously [Reference Wheeler2]. However, recent studies [Reference Müller12–Reference Thomas14] comparing the effect of using different recall periods, suggested that using long periods (e.g. 30 days) leads to underestimation of AGI incidence, probably because respondents are likely to forget episodes of illness as time passes, especially if symptoms are mild. Possible misclassification of AGI due to non-infectious causes could also have inflated our estimates.
The representativeness of our study could have been limited by a bias in selection, although the demographic features of the sample were highly comparable to those of the general population. Coverage bias in landline telephone surveys may lead to under-representation of specific population subgroups such as patients in hospitals, residents of rest homes or other community settings, homeless and low-income subjects with no access to private landlines, as well as singles, students and migrants who for a number of reasons (cost, frequent change of abode, little time spent at home) possess only mobile phones. Data from the Eurobarometer study [6] showed that, in 2009, 32% of Italian households were mobile phone-only users, a higher proportion than the average for other EU countries (25·0%). Although the mobile phone-only population typically differs significantly from the general population with respect to key socio-demographic characteristics, in particular age and marital status, a recent study found that the effect of the coverage bias due to high mobile-only phone and low no-phone rate in the population in Italy was considerably lower than in other countries with similar phone-use patterns [Reference Busse and Fuchs15]. Neverthless, the consequence of both coverage and sampling bias on the estimates of the burden of AGI may be not negligible, as different AGI incidence rates compared to other population groups were observed for some of these groups, such as students and immigrants.
The relatively low response rate (39·5%), although comparable to those reported in other studies [Reference Majowicz10, Reference Sargeant, Majowicz and Snelgrove16], could also have biased our results.
The distribution of the incidence of AGI in the population had the same gender-age specific pattern observed in other studies [Reference Majowicz10, Reference Majowicz17–Reference Thomas20], with a higher incidence in females and in children. The negative trend in the incidence towards the elderly has also been previously reported [Reference Müller12, Reference Gauci21]. Several reasons can be suggested to explain these findings. Children may have a higher susceptibility to enteric pathogens even at low infectious doses, on account of low levels of immunological protection, and are also exposed to pathogens specific to their age, such as rotavirus. Additionally, the particular behaviour of children favours exposure to enteric pathogens, especially in community settings such as day-care centres and schools.
The higher propensity of women to develop AGI is consistent with many other studies, although a contrasting pattern has also been described in Denmark [Reference Müller12], Malta [Reference Gauci21] and Cuba [Reference Aguiar Prieto22]. Some authors suggested that handling food [Reference Majowicz10, Reference Sargeant, Majowicz and Snelgrove16, Reference Gauci21] and caring for children [Reference Scallan3, Reference Thomas20, Reference Gauci21] may underlie this pattern, bringing women more frequently in contact with enteric pathogens than men. In our study, however, the higher incidence rate of AGI in women only became apparent from the 10–24 years age group. The role of such factors in this group appears only marginal, suggesting that other reasons, such as biological factors, may play a role.
The incidence of AGI characterized by diarrhoea was influenced by season, with strong differences observed between children aged <5 years and the rest of the population. These patterns could reflect a different distribution of the aetiological agents of AGI in the population throughout the year and in specific age groups. The winter peak of diarrhoeal illness observed in young children could be associated with an increased number of viral infections, especially rotavirus, which are characterized by diarrhoea and, less frequently, by vomiting and have a typical seasonal trend. This is consistent with observations from the European rotavirus infection surveillance network (EuroRotaNet) for the period 2005–2008, which showed that in Italy infections occur mostly between January and April, while they are rare during the rest of the year [Reference Iturriza-Gómara23]. The smaller increase in cases of diarrhoea observed in late summer could have been associated with bacterial infections such as salmonellosis or campylobacteriosis, which usually peak in that period. Data from the Enter-net Italia laboratory surveillance for the period 2007–2009 [Reference Luzzi24] show that the highest number of infections associated with Salmonella spp., Campylobacter spp. and Verocytotoxin-producing E. coli (VTEC) were reported between August and September.
The incidence rate of AGI characterized by vomiting alone was higher than that for cases of diarrhoea and showed a fairly constant trend over the months. As most infectious agents responsible for AGI are reported to be highly seasonal [25] it is possible that many of the cases of vomiting alone were due to non-infectious causes. This might cover other underlying patterns associated with infectious agents, such as the winter peak of norovirus infections which, surprisingly, was not observed in our study. However, it is possible that cases of norovirus were included in those in which both vomiting and diarrhoea were reported, as norovirus frequently also causes diarrhoea [Reference Medici26].
The impact of AGI on the healthcare system and the associated social costs appears relevant, with 1/3 cases seeking medical care and/or reporting a loss of working or school days, and 1/2 taking medication. In our study the proportion of respondents who consulted physicians, even if only by telephone, was considerably higher than in most previous studies. We believe that this behaviour depends largely on the way the public national healthcare system is organized in Italy. Visits to a doctor or primary healthcare unit are free for everybody, and only a small fee is payable towards the costs of laboratory tests or medication prescribed by a national health service physician. Another possible reason for the high rate of recourse to physicians is the requirement of a certificate to justify absence from work or school. All employees are required to justify any absence due to health reasons. Similarly students attending school (from nursery school to high school) have to provide certificates in cases of illness lasting ⩾5 days.
Notwithstanding the major differences in organization of the Italian and US national healthcare systems, the factors that contributed most to the recourse to medical care were similar to those reported in the USA by Scallan et al. (young age of patient, duration of illness >3 days, presence of fever) [Reference Scallan27].
From the epidemiological perspective, the large number of respondents who consulted a physician should potentially prove useful in the high-sensitivity surveillance of AGI. However, this usefulness is markedly reduced by the extremely low number of AGI cases for which, based on our estimate, a confirmed laboratory diagnosis is available. The notification rate of salmonellosis in Italy in 2007 (21·8 cases/100 000) [28] was lower than the overall mean notification rate reported from the EU (34·1 cases/100 000) [25], suggesting that the low sensitivity of the Italian reporting system may have contributed to this difference.
In conclusion, although different sources of bias could have limited the efficacy of our study in providing an overview of the occurrence of AGI in the Italian population, our survey nonetheless provides a contribution to a comprehensive estimate of the global burden of foodborne AGI. Our results suggest that the international standard case definition of AGI [6] may lead to the identification of a large proportion of AGI cases possibly associated with non-foodborne pathogens. We thus agree with the conclusion of Hall et al. [Reference Hall9] about the need to consider this issue carefully when investigating foodborne illnesses using a syndrome-based definition of AGI.
The contribution of our study to the overall goal of reducing the socioeconomic burden of foodborne AGI in Italy, which is essentially preventable, lies mainly in the estimate of the annual incidence rate and in the characterization of the population at greatest risk. This information, integrated with that deriving from outbreak investigations and laboratory data on the implicated aetiological agents could help to evaluate the contribution of different sources to the global burden of foodborne illness. Finally, the results of our study could be pooled with those from parallel surveys conducted with a similar methodology in Europe [Reference Baumann-Popczyk11, Reference Müller12] to derive regional estimates of the burden of AGI.
ACKNOWLEDGEMENTS
The study was partly supported by the European Community Network of Excellence MedVetNet (contract no. FOOD-CT-2004- 506122) and co-funded by the Italian Ministry of Health – Italian Centre for Disease Control (CCM). The authors thank Arie Havelaar for coordinating the activities of MedVetNet WP23 on ‘Foodborne pathogens prioritization’, Pawel Stefanoff for coordinating the parallel studies and supporting development of the protocol, Antonino Bella for the statistical analyses, Ida Luzzi for providing data from the Enter-Net Italia surveillance and Franco Ruggeri for his advice and expertise in the interpretation of data.
APPENDIX
Formulae for calculating prevalence and incidence


Upper and lower 95% confidence limit for incidence:
where n=total number at risk and x=number of cases.
where x=mean duration of illness.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
DECLARATION OF INTEREST
None.









