INTRODUCTION
Acinetobacter baumannii is an opportunistic pathogen and is emerging as a cause of numerous global outbreaks in hospitals [Reference Bogaerts1, Reference Bou2] and several widespread strains of the species are increasingly resistant to many classes of antibiotics. Carbapenems have been widely used to treat serious multidrug-resistant A. baumannii infections; however, carbapenem-resistant strains are commonly reported worldwide [Reference Perez3], raising serious concerns about the limited antimicrobial treatment options remaining.
Resistance to carbapenems in A. baumannii occurs due to the accumulation of various resistance mechanisms including β-lactamase production, the loss of outer-membrane protein (OMP) and/or overexpression of efflux pumps or more rarely with penicillin-binding protein alterations [Reference Poirel and Nordmann4]. There are few reports of combinations of different resistance mechanisms in single isolates of A. baumannii, but Bratu et al. showed a correlation of antimicrobial resistance with β-lactamase production and porin and efflux pump, but this was not limited to carbapenem-resistant strains of A. baumannii [Reference Bratu5].
There are marked geographical differences in the prevalence of the various mechanisms of carbapenem resistance in A. baumannii. Various different carbapenemases, for example OXA-58 oxacillinase in Turkey and VIM-2 in South Korea, have been described in Asia and Europe [Reference Poirel and Nordmann4, Reference Gur6, Reference Lee7]. Here, we evaluated the epidemiology of carbapenem-resistant A. baumannii (CRAB) infections in a Korean hospital and investigated various mechanisms of carbapenem resistance in isolates and the effect of combinations of mechanisms on the degree of carbapenem resistance. In addition, we compared the clinical characteristics, outcomes and genotypic analyses of the carbapenem-resistant mechanism of A. baumannii in infected vs. colonized patients.
MATERIALS AND METHODS
Patient selection and case definition
From January to June 2009, a total of 105 non-repetitive A. baumannii strains in clinical samples were collected from a 1200-bed tertiary hospital in Korea. Demographic characteristics, various risk factors and outcome were compared between patients classified as infected or colonized. Risk factors included comorbidity, prior admission to hospital in the preceding month, prior surgery, the length of stay before isolation of A. baumannii, APACHE (acute physiological and chronic health evaluation) II score, ventilator support, recent invasive procedures, and prior antibiotic therapy in the month before isolation of A. baumannii.
Infection was assessed according to Center for Disease Control (CDC) criteria, and defined by the isolation of A. baumannii from a sterile site in patients with definite clinical signs of infection [Reference Garner8]. Colonization was defined as the growth of A. baumannii from sputum, open wounds or other non-sterile sites, without clinical signs or symptoms of infection. Approval for the study was obtained from the institutional review boards of our hospital.
Bacterial strains and antimicrobial susceptibility testing
Species were identified using the VITEK® 2 system (bioMérieux, France). The bla OXA-51-like gene was detected by PCR to confirm species of A. baumannii [Reference Turton9]. Minimum inhibitory concentrations (MICs) were determined by agar dilution for the following antimicrobial agents: amikacin (Donga Pharmaceutical Co., Korea), cefepime (Boryung Pharmaceutical Co., Korea), piperacillin (Yuhan Co., Korea), ceftazidime (SK Chemical Co., Korea), ampicillin/sulbactam (Shinpoong Co., Korea), levofloxacin (Ildong Pharmaceutical Co., Korea), imipenem (Choongwae Pharmaceutical Co., Korea), meropenem (Yuhan Co.), tigecycline (Wyeth Pharmaceuticals Inc., USA) and colistin (Sigma Aldrich, USA). Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213 and Pseudomonas aeruginosa ATCC 27853 were used for quality assurance. Antimicrobial susceptibility was interpreted according to Clinical and Laboratory Standards Institute guidelines [10], except for tigecycline for which susceptibility/resistance breakpoints were interpreted according to the US Food and Drug Administration criteria (susceptible ⩽2 mg/l, intermediate 4 mg/l, resistant ⩾8 mg/l) [11]. Seventy-five meropenem or imipenem non-susceptible, including intermediate resistant, A. baumannii strains were selected for further study.
DNA typing
ApaI digests of genomic DNA from strains were resolved by pulsed-field gel electrophoresis (PFGE) as described previously [Reference Seifert12]. Cluster analysis by the unweighted pair-group method with mathematical averaging was performed using the Dice coefficient and a similarity cut-off of ⩾70% on the dendrogram was used to define related strains within a cluster.
Detection of OXA genes, and genes encoding metallo-β-lactamases
Detection of the four main groups of OXA-carbapenemase genes (bla OXA-23-like, bla OXA-24-like, bla OXA-51-like, bla OXA-58-like) was performed using a previously described multiplex PCR assay [Reference Woodford13]. The insertion sequence ISAba1 upstream of the bla OXA-23 and bla OXA-51 genes was sought using combinations of the ISAba1F primer and the OXA23-R/OXA51-R primer [Reference Turton14]. PCR analysis was performed to confirm the presence of MBL genes with primers specific for the bla IMP, bla VIM, bla SIM, bla SPM-1 and bla GIM-1 genes [Reference Ellington15]. Searches and alignments for the nucleotide sequences were performed with the BLAST program (http://www.ncbi.nlm.nih. gov/BLAST).
Analysis of and amplication of carO gene
OMPs of strains were analysed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) by a standard protocol [Reference Carlone16]. A. baumannii ATCC 19606 was used as a positive control for CarO expression. Target mRNA was transcribed into cDNA using oligo(dT)15 primer and optiscript reverse transcriptase (Intron, Korea). PCRs were performed with a 50 μl reaction mixture containing 10 mM Tris–HCl (pH 8·4), 50 mM KCl, 1·5 mM MgCl2, 200μM of each deoxynucleoside triphosphate, 1 μm each of forward (5′-CAT ATG AAA GTA TTA CGT GTT TTA GTG -3′) and reverse (5′-GGT ACC TTA CCA GTA GAA GTT TAC ACC-3′) primers, 50 ng target cDNA and 2 U Taq polymerase (Enzynomics, Korea). An initial denaturation step at 94°C for 3 min was followed by 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C with a final extension step of 10 min at 72°C. The carO gene product was sequenced and homology searches in the GenBank database were performed using the BLAST program.
Detection of efflux pump
To determine the presence of efflux mechanism, strains were tested for susceptibility to carbapenems by the agar dilution method with Mueller–Hinton agar plates with and without 100 μg/ml of the efflux pump inhibitor, 1-(1-naphthylmethyl)-piperazine (NMP, Sigma, USA) [Reference Bratu5, Reference Pannek17]. The positive criterion for phenotypic detection of efflux pump was at least a fourfold reduction in the MICs of carbapenem in the presence of NMP [Reference Shi18]. Efflux pump-encoding genes adeA, adeB and adeC, and regulatory genes (adeR, adeS) were detected with primers as described previously [Reference Lin, Ling and Li19].
Statistical analysis
All statistical analyses were performed using SPSS software, version 13·0 (SPSS Inc., USA), and a P value <0·05 was considered statistically significant. Statistical significance was assessed via χ2 test or Fisher's exact test for categorical variables and Student's t test or the Mann–Whitney U test for continuous variables. Risk factors associated with carbapenem non-susceptible A. baumannii infection were analysed using logistic regression analyses. Variables with a P value <0·1 in the univariate analyses were included in a logistic regression model for multivariate analysis.
RESULTS
Characteristics of bacterial isolates
We collected 105 clinical isolates of A. baumannii, of which 75 (71·4%) were intermediate or resistant to carbapenems. These strains were recovered from sputum (47), wound (11), catheter tip (6), blood (5), bile/intra-abdominal drainage (4) and urine (2); 49 were from patients in intensive-care units (ICUs), and the remainder from patients in general wards.
Clinical characteristics of patients with CRAB
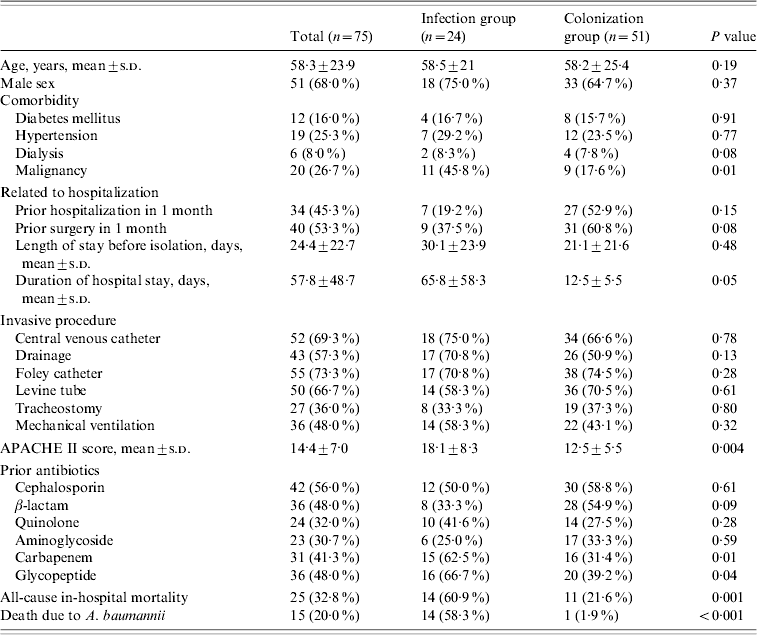
A comparison of the clinical characteristics and outcomes of the 75 patients colonized or infected with CRAB is shown in Table 1. The mean age was 58·3±23·9 years and 68% were male. Twenty-four patients were defined as infected and 51 patients as colonized. More patients with infection had malignancies and longer duration of hospital stays as well as higher APACHE II scores. The use of carbapenems and glycopeptides prior to A. baumannii isolation was more frequent in the infected group and both the all-cause in-hospital mortality and the A. baumannii-attributable mortality were higher in this group. The presence of invasive devices such as central venous catheter, urinary catheter, or surgical drainage was not a significant risk factor for A. baumannii infection. Univariate analysis showed that the risk factors associated with infection were malignant disease, duration of hospital stay, APACHE II score, and prior use of carbapenems and glycopeptides. Of these, malignancy as underlying disease and high APACHE II score (>15) were significantly associated with A. baumannii infection on multivariate analysis (P< 0·05).
Table 1. Clinical characteristics and outcomes of patients with A. baumannii
Antimicrobial susceptibility
The susceptibility patterns of the 75 A. baumannii strains are shown in Table 2. Most exhibited resistance (>95%) across different classes of agents notably ampicillin/sulbactam, ceftazidime, levofloxacin and piperacillin. Six isolates showed carbapenem discordant susceptibility (intermediate or resistant to meropenem, but susceptible to imipenem). All strains were susceptible to colistin but 38·7% were intermediate or fully resistant to tigecycline.
Table 2. Susceptibility patterns of 75 carbapenem non-susceptible A. baumannii strains
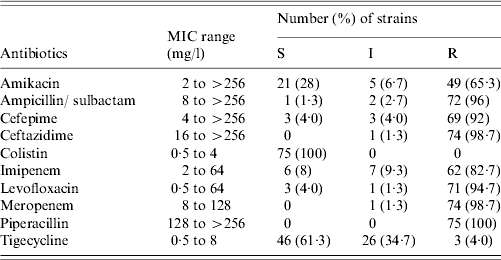
MIC, Minimum inhibitory concentration; S, susceptible; I, intermediate resistant; R, resistant.
DNA typing
Eighteen distinct clusters of strains were identified by PFGE. Four clusters (E, I, L, N) accounted for 64% of the strains with pattern E represented by 27 strains (Fig. 1).
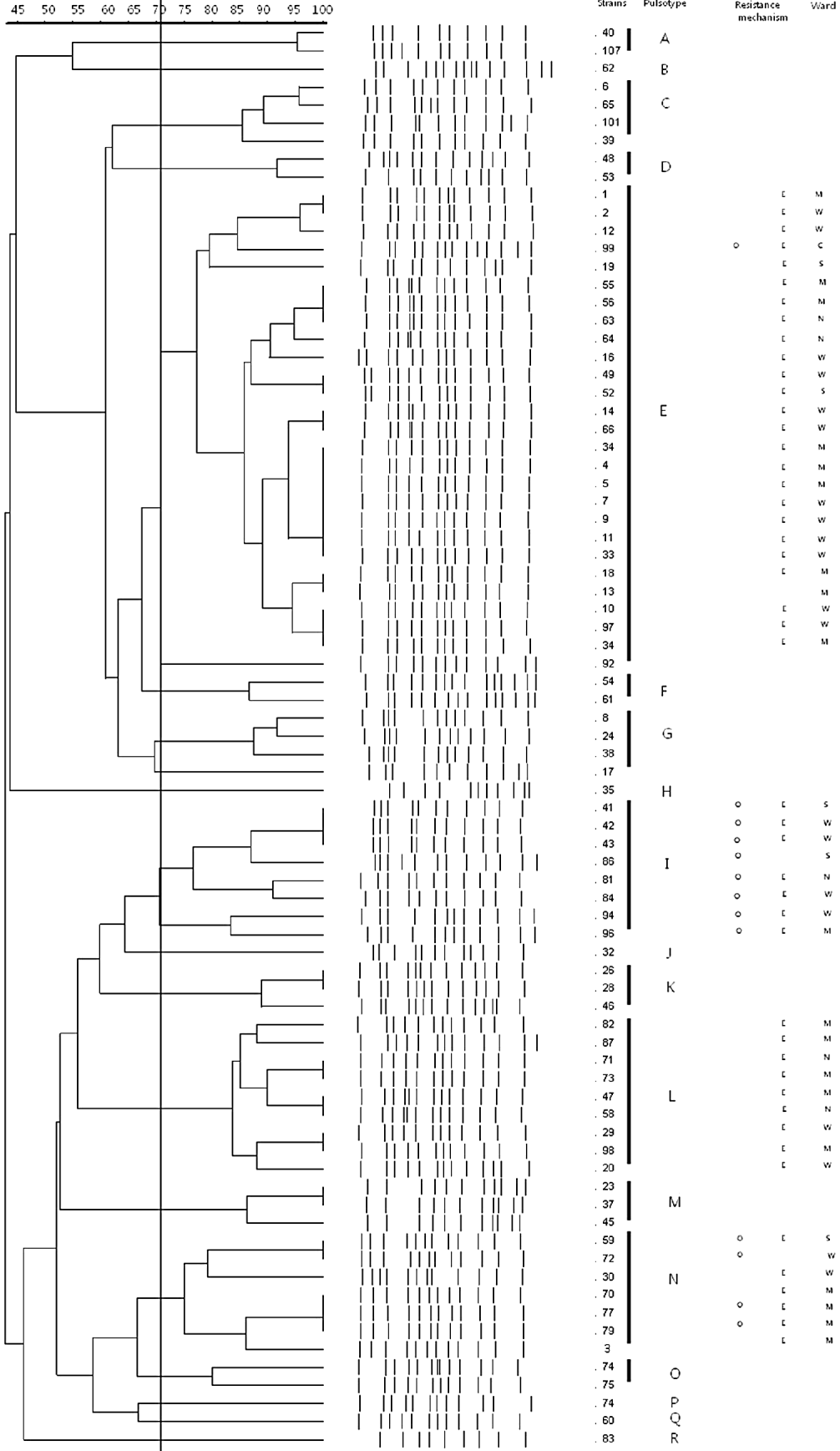
Fig. 1. Dendrogram of PFGE types of carbapenem-resistant A. baumannii strains. Strains producing ISAba1-enhanced bla OXA-23-like (O); efflux pump overexpression (E); surgical intensive-care unit (ICU) (S); medical ICU (M); neurosurgery ICU (N); coronary care unit (C); general wards (W).
Resistance mechanisms
All strains carried the OXA-51-like gene characteristic of A. baumannii, and among them 46 isolates had ISAba1 upstream of the bla OXA-51-like genes. The bla OXA-23-like genes were carried by 28 strains preceded upstream by ISAba1. Coexistence of bla OXA-23-like and ISAba1-activated bla OXA-51-like genes was detected in three strains. Only one strain harboured bla VIM-like genes, and sequencing of bla VIM amplicons identified a bla VIM-2 allele. All strains were negative for the other MBL genes (bla IMP, bla SPM, bla SIM, bla GIM).
Five strains did not yield a carO PCR product with a size of 750 bp, and of these three gave a product of ~1700 bp. The carO sequence of A. baumannii ATCC 19606 was identical to GenBank accession no. FJ652396.1, which was used as a positive control. The ~1700 bp DNA sequence of the PCR fragment corresponding to the ISAba825-interrupted carO gene was deposited in the GenBank database under accession no. AY751532. SDS–PAGE of the OMP profiles of the five strains confirmed the absence of a protein of ~25 kDa.
PCR amplication showed that 74 strains were positive for adeA and adeB genes and all but one carried adeC, adeR and adeS genes. The efflux pump inhibition test using NMP showed that the meropenem MICs decreased by fourfold or greater in 56 strains. Of the 69 imipenem non-susceptible A. baumannii strains, the imipenem MICs decreased by a similar margin in 58 strains.
Influence of resistance mechanism
The MICs of imipenem and meropenem associated with the various resistance mechanisms of the strains are shown in Table 3. Strains carrying bla OXA-23-like genes had higher MICs for both agents than strains with bla OXA-51 (median, 32 vs. 16, respectively, P<0·05). Furthermore, the coexistence of multiple resistance mechanisms did not lead to higher carbapenem MICs than those of strains with a single mechanism. Nine mechanisms of resistance were identified but there was no significant difference in mechanisms of strains from infected and colonized patients. Coexistence of ISAba1-activated bla OXA-51-like genes and overexpression of efflux pump were the most prevalent mechanisms in both groups (54·2% vs. 41·2%, respectively).
Table 3. Effect on carbapenem minimum inhibitory concentrations (MICs) of resistance mechanism in A. baumannii
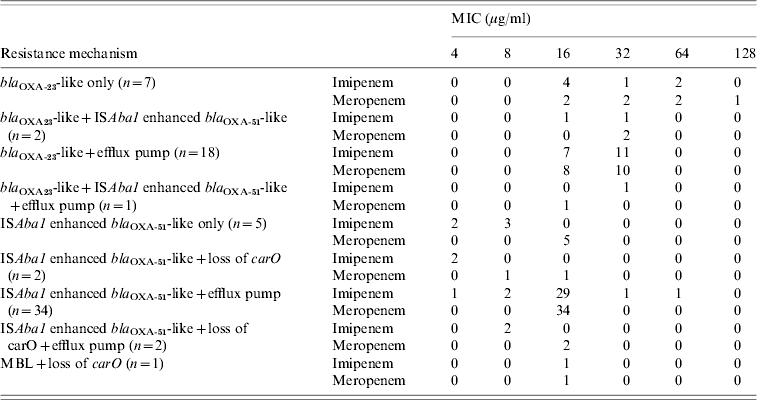
bla OXA-23-like was consistently associated with ISAba1.
DISCUSSION
A. baumannii is usually a healthcare-associated pathogen, particularly in the subset of critically ill patients in ICUs. The emergence of CRAB in the hospital setting is a serious problem worldwide, mainly because of limited therapeutic options for treating this infection. There are several factors that lead to the emergence of multidrug resistance in A. baumannii and include the acquisition of class D carbapenem-hydrolysing oxacillinases and/or class B metallo-β-lactamases, decreased membrane permeability due to loss of porins, and multidrug efflux systems [Reference Poirel and Nordmann4, Reference Munoz-Price and Weinstein20–Reference Siroy23].
Our study showed that multiple factors contribute to carbapenem resistance in A. baumannii, and the most dominant was ISAba1 preceding OXA-51, producing strains with overexpression of efflux pump, followed by OXA-23 with overexpression of efflux pump. OXA-51-like enzymes are capable of hydrolysing carbapenems but their activity is extremely weak and requires an insertional element as a strong transcriptional promoter [Reference Poirel and Nordmann4, Reference Turton14]. Forty-six and 28 of the 75 carbapenem-resistant strains studied here carried respectively ISAba1-activated bla OXA-51-like and bla OXA-23-like genes. The bla OXA-23-like genes have been documented in strains associated with outbreaks of CRAB in Asia, Europe and South America [Reference Poirel and Nordmann4, Reference Gur6, Reference Mugnier24, Reference Villegas25], and the presence of IS elements upstream of this gene may also play a role in gene expression by providing promoter sequences [Reference Poirel and Nordmann4].
There is limited information on the role of OMPs in multidrug-resistant A. baumannii; however, resistance to carbapenems has been associated with the loss of a 29 kDa OMP, designated as CarO [Reference Mussi22, Reference Mussi, Limansky and Viale26], and ISAba1 is reported to cause insertional inactivation of CarO. We showed disrupted expression of carO in five strains and the loss of an OMP of ~25 kDa in these strains compared to a positive control [Reference Siroy23]. However, further research on the outer membrane permeability in CRAB is necessary.
A. baumannii possesses an efflux system, adeABC, that belongs to the resistance-nodulation-division family of transporters [Reference Wieczorek27, Reference Magnet, Courvalin and Lambert28]. An increased level of adeABC expression has been shown to correlate with a reduced level of susceptibility to gentamicin or tigecycline [Reference Wieczorek27–Reference Peleg, Adams and Paterson29]. We demonstrated that the addition of an efflux pump inhibitor (NMP) led to a fourfold or greater reduction in the MICs of meropenem and imipenem [57/75 (76%) vs. 59/69 (85·6%), respectively], which suggests but does not confirm that an efflux mechanism may be involved in carbapenem resistance in this species. However, we did not determine the expression level of adeB by RT–PCR, and did not confirm that the observed reduction of carbapenem MICs in the presence of the inhibitor was associated with expression of adeABC or the regulatory genes. Indeed, sequencing of adeRS revealed several point mutations which did not correlate with adeABC (data not shown).
Our data indicate that the presence of multiple mechanisms in strains such as production of carbapenemase, loss of CarO and efflux pump overexpression did not result in increased carbapenem resistance compared to strains with a single mechanism. It appears that ISAba1-activated bla OXA51-like enzymes have weaker hydrolytic activity than enzymes encoded with bla OXA-23, but no direct evidence for this is provided.
Several case-control studies have identified risk factors associated with the acquisition of A. baumannii, and these include prolonged hospitalization, ICU admission, recent surgical procedures, antimicrobial agent exposure, central venous catheter use and prior hospitalization [Reference Maragakis and Perl30–Reference Jang33]. However, most of these studies lacked a standard methodology, and designated carbapenem-susceptible A. baumannii or non-A. baumannii as a control group [Reference Baran31, Reference Lautenbach32, Reference Lee34]. We determined that the risk factors for infection in patients with CRAB were malignancy as an underlying disease and high APACHE II score and showed that resistance mechanisms did not differ between infected and colonized groups. Molecular typing showed that although one clone accounted for about one-third of the strains, the remainder exhibited considerable diversity. Further study using multi-locus sequence typing may allow us to compare the prevalent PFGE types with those from epidemics worldwide.
The main limitation of this study is that it was confined to a single centre and it would be valuable to extend the geographical origin of the strains. In addition, we were unable to follow patients colonized with A. baumannii who went on to develop infection; however, we tried to overcome this bias by including strains from distinct individuals.
In conclusion, CRAB bearing a class D carbapenemase, and OXA-51-like or OXA-23-like enzymes, are relatively widespread in a tertiary hospital in Seoul, Korea and various factors, including loss of OMP or efflux pump, probably contribute to carbapenem resistance. Multiple resistance mechanisms did not confer higher resistance and the underlying clinical status of patients may play a more important role in the development of CRAB infection.
DECLARATION OF INTEREST
None.






