INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) has a high prevalence in acute-care hospitals in Spain, and also in long-term-care facilities (LTCFs) [Reference Dominguez1–Reference Olona-Cabases3]. This scenario is similar to other countries of the European Union [Reference Cretnik4–Reference von Baum8]. In the nosocomial setting patients with persistent MRSA carriage have a higher risk of developing MRSA infections [Reference Pujol9, Reference Corbella10] than methicillin-susceptible S. aureus (MSSA) carriers and non-carriers. Although, it appears that MRSA colonization in LTC settings might have different clinical implications than in acute-care hospitals, few studies have addressed this issue and most report a low prevalence of MRSA infection in residents [Reference Pujol11, Reference Murder12]. A relatively small number of residents require hospitalization or die as a consequence of MRSA infections and this suggests that severe infections are uncommon in this population. The most frequent MRSA infections in LTCFs are skin and soft tissue infections while bloodstream infections account for about 10% of cases [Reference Bradley13].
Very few longitudinal studies have investigated the incidence of MRSA infections in residents in LTCFs [Reference Murder12, Reference Stone14]. We therefore considered it necessary to analyse the clinical impact of MRSA colonization in this population in order to identify suitable measures to prevent spread and infections due to MRSA in this setting. To this end a multicentre longitudinal study was performed among residents in community LTCFs to determine the incidence of MRSA infection and assess whether MRSA colonization is associated with greater risk of infection and overall mortality.
METHODS
Study population and characteristics of community LTCFs
Characteristics of this population have been described previously [Reference Manzur2]. Nine community LTCFs for the elderly, located in two communities in Spain (Catalonia and Balearic Islands) with 1586 beds (median 120, range 72–552) were included. Five were located in the catchment area of a 900-bed acute-care hospital (Hospital Universitari de Bellvitge), three in that of a 490-bed hospital (Corporació Sanitària Parc Taulí) and one in that of an 800-bed hospital (Hospital Universitari Son Dureta). These facilities provide care for the elderly long-term resident, who may be disabled or infirm. Each LTCF has a dementia ward and its own medical staff. Residents are accommodated in rooms with up to three beds. Surveillance for MRSA and decolonization procedures are not routine. In addition to standard precautions for all patient care, contact precautions are applied for residents colonized or infected by MRSA. Known MRSA carriers are not denied admission [Reference Rodríguez-Baño15].
Study design
This was a multicentre prospective cohort study conducted from November 2005 to May 2007. The studied population consisted of all residents in the LTCFs at baseline (n=1377) and was identical to that of our previously published cross-sectional study [Reference Manzur2]. A total of 231 residents was found to be colonized with MRSA at baseline (MRSA carriers cohort). A representative sample of non-MRSA carriers was selected from the 1146 residents without MRSA at baseline as follows: for each MRSA carrier identified, one non-MRSA carrier was randomly selected from the same ward and screened 6 months later. Those with two consecutive negative cultures were included in the non-MRSA carrier cohort (n=196). Subjects were visited by the investigators every 6 months over an 18-month period.
During this period no changes were made to infection control practices in the LTCFs and data from the study results were not available to clinical staff. Decolonization treatment or contact precautions were not applied to the MRSA carriers detected throughout the study.
Data collection and definitions
Complete clinical data were obtained for all residents at baseline and medical charts were reviewed thereafter at 6-monthly intervals. Occurrences of infections, decubitus ulcers, antibiotic use, hospital admission and deaths were recorded. In the MRSA carrier cohort, persistent colonization was defined as at least two MRSA-positive cultures separated by fewer than two negative cultures. Transient colonization was defined as two or more negative cultures after a single positive culture for MRSA [Reference Manzur16]. Duration of carriage was defined as the period from the first positive culture until the first negative culture with a consecutive negative culture if available and only residents who survived at the end of the study were considered for this analysis. MRSA infection was recorded in the clinical charts of each facility.
Microbiological methods
Nasal, and where applicable, decubitus ulcer swabs were obtained for culture every 6 months. Swabs were placed in Stuart's transport medium and plated on coagulase-mannitol agar plates and selective MRSA agar (MRSA Select, Bio-Rad Laboratories, Spain) before inoculating into brain heart infusion plus 7% NaCl. After 24 h of incubation at 35°C, broths were subcultured on coagulase-mannitol and selective MRSA agar; plates were incubated for 48 h and inspected daily. Putative S. aureus colonies were identified by the latex agglutination test (Pastorex® Staph-plus, Bio-Rad Laboratories) and DNase production (DNase Test Agar, Biomedics, Spain). Methicillin resistance was determined by the cefoxitin disk diffusion method and antimicrobial susceptibility testing was performed by disk diffusion following Clinical and Laboratory Standards Institute recommendations [17].
Statistical analysis
Secondary outcomes (MRSA infections and overall mortality) were compared between prevalent MRSA carriers and non-MRSA carriers. Categorical variables were analysed with χ2 or Fisher's exact tests as appropriate and continuous variables by Student's t test or non-parametric tests. Mortality was compared by the Kaplan–Meier method. All statistical tests were two-tailed and P<0·05 was deemed significant. SPSS package version 15·0 was used (SPSS Inc., USA).
Approval for the study was obtained from the Research Ethics Committee of the Hospital Universitari de Bellvitge. No written informed consent was obtained because the study met the criteria for a waiver of this requirement.
RESULTS
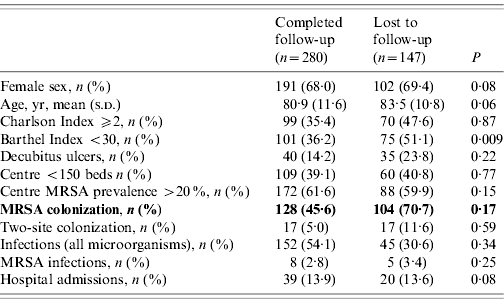
The MRSA cohort comprised 178 colonized residents (86 490 patient-days) and the non-carrier cohort 196 patients (97 470 patient-days). Over the study period, 147 residents were lost to follow-up (53 at 6 months, 60 at 12 months and 34 at 18 months), 99 residents died and 30 were discharged. Table 1 compares residents who were lost to follow-up with those followed to completion.
Table 1. Characteristics of residents who were lost to follow-up with those followed to completion for the study period
Overall 14 residents developed MRSA infections, nine in the MRSA cohort and five in the non-carrier cohort. The type of infections were: 10 skin and soft tissue infections, seven related to decubitus ulcers, one urinary tract infection, one chronic external otitis and two respiratory infections which both required hospital admission.
The incidence rate of MRSA infection in the total MRSA cohort (n=178) was 0·12/1000 patient-days and in the 196 non-carriers, 39 residents acquired MRSA colonization during the study, giving an incidence rate of MRSA infection in this cohort of 0·05/1000 patient-days. The incidence rate of MRSA infection was statistically similar for prevalent MRSA carriers and residents with newly acquired MRSA colonization (P=0·46). Table 2 shows that no difference was found in MRSA infection rate between transient and persistent MRSA carriers (lineal regression P=0·69). In addition there were no differences in infections of any aetiology for both cohorts, and MRSA carriers did not require more hospital admissions than non-carriers during the study period (Table 3). The mortality rate was 20·8% in residents in the MRSA cohort and 16·8% in non-carriers. Four of 14 residents with MRSA infection died during the study period but these were not attributed to MRSA infection. No statistical difference was found in the overall mortality in either group (log rank test 0·19, P=0·66) (Fig. 1).
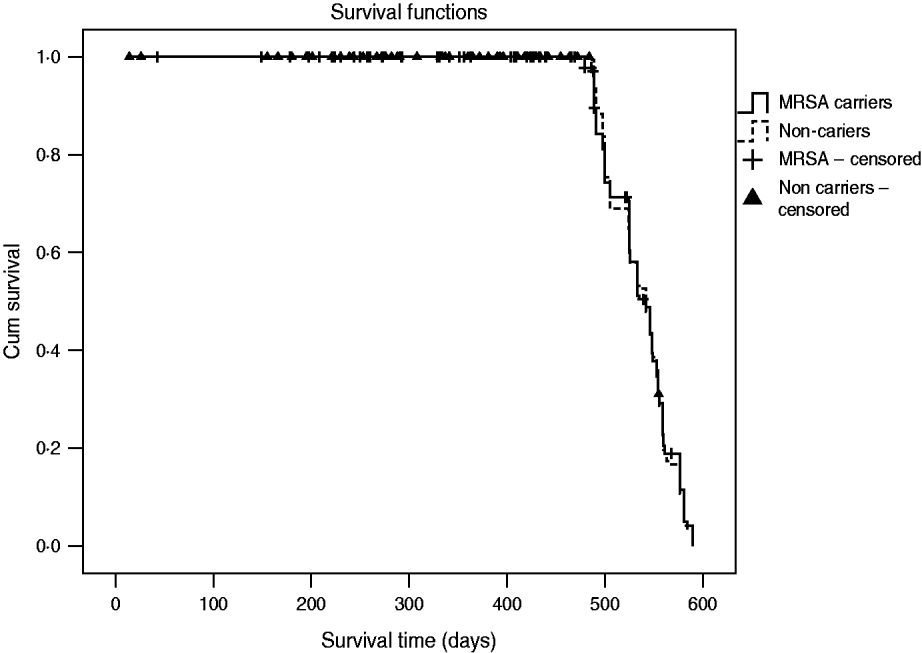
Fig. 1. Overall mortality during the study period (18 months) for cohorts of MRSA carriers and non-carriers.
Table 2. Incidence rate of MRSA infection during the 18-month period related to the duration of MRSA colonization
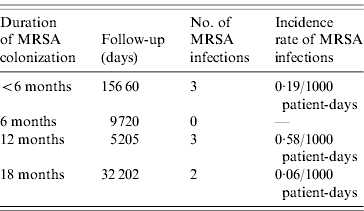
Table 3. Comparison of clinical outcomes in the MRSA-colonized cohort and the non-carrier cohort
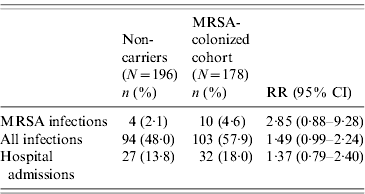
RR, Relative risk; CI, confidence interval.
DISCUSSION
In a previous study we reported a prevalence of MRSA colonization of about 17% in residents of community LTCFs in Spain [Reference Manzur2], which represents a large reservoir of this microorganism in the healthcare setting. Several studies have highlighted the relevance of this epidemiological aspect which might influence the infection control practices implemented by acute-care hospitals [Reference Cooper18–Reference Ruiz de Gopegui22] but there are limited data on the relationship of MRSA colonization and the development of infection in residents of LTCFs [Reference Murder12, Reference Stone14]. This aspect has usually been assessed in settings where patients are at great risk of MRSA infection, such as intensive care units [Reference Corbella10]. Our findings show that MRSA carriers in community LTCFs are not at high risk of developing severe MRSA infection while residing at the facility. This is in agreement with the out-of-hospital risk of MRSA infection reported in another population [Reference Datta and Huang23]. Moreover, from a clinical point of view, MRSA infections were not severe and only 2/14 cases required hospitalization. As previously reported, we found that the main MRSA infections were skin and soft tissue infections. Remarkably the majority of infections were associated with the presence of decubitus ulcers, the most frequent skin lesion in this population. This emphasizes the need to enhance efforts to prevent the development of decubitus ulcers. Since in community LTCFs accurate microbiological diagnoses are often lacking, MRSA infections could have been underestimated and therefore we analysed the incidence of infection of any aetiology in both cohorts and found no differences. Prior studies have demonstrated an incidence of MRSA infections of 6·5% [Reference Bradley13] and a relative risk of 3·6 in MRSA carriers in LTCFs [Reference Murder12].
Persistent MRSA carriers are more often colonized at multiple sites, are more likely to transmit to others, and become infected than transient carriers [Reference Vandenbergh24]. However, this aspect has not been studied in MRSA-colonized residents in LTCFs. A recent study performed in a LTCF showed that the degree of bacterial colonization in persistent MRSA carriers was significantly higher than in transient MRSA carriers [Reference Stone14]. We did not find a relationship between the incidence of MRSA infection and the duration of MRSA carriage, possibly because of the very few cases of MRSA infections. Moreover, the incidence of MRSA infection was similar in prevalent MRSA carriers and residents with newly acquired MRSA, i.e. MRSA colonization acquired while residing at the facility. A recent study, which included a small number of residents in LTCFs, found that the risk of MRSA infection in long-term carriers in the first year exceeded the risk of infection in subsequent years [Reference Datta and Huang23]. It appears that MRSA carriers remain at considerable risk for subsequent MRSA infection regardless of the time since the initial detection of MRSA carriage. Available data indicate that MRSA colonization in LTCFs may have different and less severe consequences than in acute-care hospitals. The risk of MRSA infection in the population of community LTCFs might not be related to the duration of colonization but might instead be attributable to known risks associated with MRSA infection such as hospitalization, bronchoaspiration, and the presence of decubitus ulcers or invasive medical devices. Except for ulcers and bronchoaspiration, these risks are not frequent in this population [Reference Vandenbergh24]. Reports of MRSA producing Panton–Valentine leukocidin (PVL) strains in LTCFs are increasing [Reference Raab25–Reference Kerttula27]. Since these strains might produce spontaneous infections, MRSA infection rates could potentially rise in residents in LTCFs without obvious clonal spread. None of the strains in this study was PVL positive [Reference Manzur2] and previous molecular typing had shown the presence of only two distinct clones [Reference Manzur16] one of which (CC5-MRSA-IV) has been reported as widely disseminated in Spanish hospitals [Reference Vindel28, Reference Rodríguez-Baño29].
We found no differences in overall mortality in MRSA carriers and non-carriers. The mortality rate was around 15–20% in this elderly population and bronchoaspiration was the most frequent cause of death (data not shown). Previous studies have reported an associated mortality of MRSA infections in LTCFs of 1% [Reference Bradley13], and a relative risk overall mortality rate of 2·0 in MRSA carriers [Reference Murder12]. Significantly higher mortality was associated with MRSA carriers in LTCFs only in patients with severe cognitive impairment [Reference Suetens30].
This study has some limitations, as we only performed cultures of nasal swabs and decubitus ulcers to detect MRSA colonization. A recent study demonstrated that more than half of community LTCF residents present multiple-site MRSA colonization and one-third of MRSA carriers would have been missed if only nasal swabbing had been performed [Reference Mody31]. Another limitation is the large number of residents lost to follow-up, principally because of death in an elderly population. Patients lost to follow-up had significantly more deterioration in functional status; this is expected since poor functional status is associated with death in this population [Reference Mahoney and Barthel32]. In addition this study was originally designed to describe the natural history of MRSA colonization in residents in LTCFs and to identify risk factors for being colonized with MRSA [Reference Manzur16]. The MRSA infection rate and mortality in both cohorts were considered as secondary outcomes, and thus, no specific calculations were initially performed to determine if the study had sufficient power to detect significant differences. Nevertheless, major strengths of this study are the prospective design and the fact that it includes multiple facilities with a similar profile. Moreover, MRSA infections and mortality were evaluated in a population with a high prevalence of MRSA carriage.
Community LTCFs are institutions intended for the promotion of a healthy lifestyle for elderly people, a segment of population that is growing steadily; promoting comfort, optimal social environment and preserving functional status of residents are major objectives. The profile of community LTCFs and the endemicity of MRSA in these centres with a low clinical impact for colonized residents while in the facility, make the implementation of control measures to limit MRSA spread controversial. Standard precautions for all residents should be applied routinely; barrier precautions, cohorting, decolonization and other measures should be undertaken only for controlling MRSA infection outbreaks [Reference Mody33–Reference Manzur and Gudiol36]. Our results together with the clinical experience and available literature suggest that MRSA infections are neither frequent nor severe while MRSA-colonized residents remain in a LTCF. However, when admitted to an acute-care centre, they may spread MRSA to other patients who may develop severe infections. Therefore the epidemiological impact of the reservoir of MRSA in LTCFs is more relevant than the clinical impact of this colonization for an individual resident. The present results support current recommendations to control MRSA spread in community-LTCFs [Reference Mody33–Reference Manzur and Gudiol36].
APPENDIX
Members of the Spanish Network for Research in Infectious Diseases who participated in the study: Raul Fernández, David Herrero, Rosario Casas, Eulalia Fontseca, Mónica Bota, Ricard Iniesta, Jesús Alburquerque, Catalina Andreu, Enrique Campos, Montserrat Vaqueiro, Esperança Antón, Jordi Trelis, Anna Esteve, Maria Canals, Ana Diaz, Eva Penelo, Antonio Oliver, Javier Ariza, Francesc Gudiol.
ACKNOWLEDGEMENTS
This work was supported by Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Spanish Network for Research in Infectious Diseases (REIPI RD06/0008).
DECLARATION OF INTEREST
None.






