INTRODUCTION
Campylobacters are important emerging pathogens worldwide causing a considerable health and economic burden [Reference Havelaar1]. Infections are usually sporadic and although outbreaks related to food, raw milk and untreated water have been described, they are unusual. Outbreaks in treated public water supplies are rare and only three have been previously documented, two in the United States [Reference Vogt2, Reference Sacks3] and one in Sweden [Reference Andersson, de Jong and Studahl4].
In September 2000, 15 cases of gastroenteritis due to Campylobacter jejuni were reported amongst residents of an economically deprived housing estate located in the South Wales Valleys. The estate, comprising mainly local government-owned rented accommodation, is located on a steep hill and is surrounded by agricultural pasture land. Cases became ill between 17 and 24 September and only low numbers of C. jejuni (<5) had been notified for the area in the 6 months prior to the outbreak indicating an unusual event. Initial investigation by the local government environmental health department found that patients were clustered in the upper part of this housing estate and reported drinking large quantities of tap water.
Chlorinated mains water is supplied to the upper part of the hillside estate (442 properties) by pumping from the main supply to a small concrete service reservoir, submerged into the hillside. The lower part of the estate (248 properties) is supplied via a different route. This raised suspicion that the geographical clustering of cases in the upper estate may have been related to water supply.
METHODS
Cohort study
We conducted a retrospective cohort study of diarrhoeal illness in estate residents in all 690 households. Information was collected by postal questionnaire on individual's self-reported exposure to household pets and farm animals. An individual dietary history was recorded, including specific questions on meats, meat products, takeaway meals, milk, carbonated drinks, tea/coffee, tap water and bottled water. Residents were asked where they purchased milk. Where home delivery of milk was recorded, the dairy supplying the milkman was identified by follow-up investigation. Residents were assigned a source of mains tap water by whether they were resident in the upper or lower estate. Any history of diarrhoeal illness amongst residents or pets was noted and dates and symptoms recorded. A case was considered to be an individual residing on the estate, with diarrhoeal illness (⩾3 stools within 24 h) lasting over 1 day in September 2000.
Nested case-control study
Using data collected from the cohort study we also carried out a nested case-control study of the 15 laboratory-confirmed cases and 93 estate resident controls that had not experienced diarrhoeal illness (simple random sample of 10% of cohort). There were no other exclusion criteria.
Statistical analysis
Univariate analysis was performed by Mantel–Haenszel χ2 test or Fisher's exact test (two-tailed) using Epi-Info, version 6 (CDC, Atlanta, GA, USA). Univariate analysis was repeated and multivariate analysis carried out by logistic regression using stata software, version 6 (Stata Corp., College Station, TX, USA) with confidence intervals adjusted for household clustering using the Huber–White robust estimate of variance. Initially all exposures were controlled for age and sex. In the second model, all exposures were controlled for age, sex, household clustering and any variables statistically significantly associated with illness at the 5% level from the first model (water supply, water consumption, milkman delivery, cold takeaway consumption and puppy/kitten in house with or without diarrhoea).
Microbiological investigations
Serotyping of campylobacter isolates was not routinely carried out by local laboratories. However, as part of the outbreak investigation samples from cases were forwarded by the two local hospital laboratories to the Laboratory of Enteric Pathogens, Central Public Health Laboratory, London (now Centre for Infections, Health Protection Agency) for serotyping.
Environmental investigations
The service reservoir outlet supplying the upper estate is sampled weekly by the water company on random days. Bacteriological and chlorination records of weekly sampling of the service reservoir were provided by the water company and evaluated by the outbreak control team. Sampling of the service reservoir supplying the upper estate was carried out daily from 30 September by the water company and samples were tested for indicator organisms (coliforms and E. coli) and free and total chlorine levels. Additional analyses for faecal streptococci and clostridia were also performed. All methods and standards used were as set out by the European Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption [5].
The water company conducted a physical external inspection of the reservoir on 2 October and one compartment of the reservoir was taken out of service for internal inspection. The second compartment was taken out of service for inspection on 11 October. Rainfall records for the area, maintained by the national Meteorological Office, were obtained.
RESULTS
Cohort study
Questionnaires were received from 528 households (76·5%) with 1220 personal responses. Diarrhoeal illness was reported by 281 (23%) of respondents. Of those, 22 (7·8%) reported blood in stools and 102 (36·3%) contacted their family practitioner. The median length of illness was 4 days (mode 2 days) and mean daily stool frequency on the worst day of illness was 10 (mode 5). The attack rate for those resident in the upper estate was 232/802 (29·0%) compared with 49/413 (11·9%) for those in the lower estate [relative risk (RR) 2·44, 95% confidence interval (CI) 1·83–3·24, P<0·001]. An epidemic curve for diarrhoeal illness in estate residents (Fig. 1) indicated raised levels of diarrhoeal illness in upper estate residents during a 2-week window in September 2000 against a general background of low levels of diarrhoeal illness in the whole estate. Attack rates varied by age, with the highest attack rate in the ⩽4 years age group (40%) and the lowest in those aged ⩾65 years (13·4%).
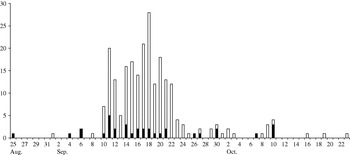
Fig. 1. Epidemic curve for diarrhoeal illness in upper (□) and lower (■) estate residents.
Upper estate residence and tap water consumption were significantly associated with illness (Table 1) with a significant dose–response (Fig. 2). The odds ratio for illness after consumption of 1–3 glasses of upper estate tap water in 24 h was 2·42 (95% CI 1·59–3·70), with increasing odds ratios for increased levels of consumption to a maximum of ⩾11 glasses (OR 18, 95% CI 3·51–92·40). The population attributable risk (PAR) for upper estate water supply was 49% and for tap water consumption 50%.
Table 1. Self-reported diarrhoeal illness in 1220 estate residents: univariate analysis by χ2 test

PAR, Population attributable risk; RR, relative risk; AR, attack rate; OR, odds ratio; CI, confidence interval.
Other exposures associated with illness were: consumption of cold milk, fizzy drinks, cold takeaways for re-heating at home, home delivery of milk by a milkman, having a puppy or kitten in the house and having a puppy or kitten with diarrhoea (Table 1).
Of 231 individuals who had milk delivered at home 71 had experienced bottle-tops being pecked by birds (30·7%). This exposure was not significantly associated with illness (RR 0·85, 95% CI 0·57–1·40, P=0·617).
Stratified univariate analysis of milk consumption among persons reporting milkman delivery was performed. There was no significant association between consuming milk and illness (OR 1·57, 95% CI 0·61–4·04, P=0·351).
Upper water supply (OR 2·97 95% CI 1·83–4·8, P<0·0001) and tap water consumption (OR 3·24 95% CI 2·18–4·8, P<0·0001) remained significant effects after controlling for other exposures (Table 2). However, one confounding factor was attendance at the estate primary school, which, although located in the lower estate received upper water supply. Many children aged between 4 and 11 years from both the upper and lower estate attended this school so that children living in the lower water supply area drank upper supply water during school hours.
Table 2. Self-reported diarrhoeal illness in 1220 estate residents: multivariate analysis by logistic regression

OR, Odds ratio; CI, confidence interval.
Being aged <11 years showed increased risk of illness independent of residence in the upper or lower estate (RR for lower part of the estate 3·48, 95% CI 2·07–5·85, P<0·001; RR for upper estate 1·70, 95% CI 1·35–2·14, P<0·001). Consumption of cold takeaway meals re-heated at home was also significantly associated with illness but only 13 of the 281 possible cases had been exposed to these (PAR 2·3%, OR 3·45, 95% CI 1·47–8·06, P<0·004). Having home delivery of milk was also associated with illness but, again, would only account for a small proportion of diarrhoeal illness in residents (PAR 6·5%, OR 1·58, 95% CI 1·03–2·44, P<0·037).
Nested case-control study (Table 3)
All laboratory-confirmed cases resided in the upper estate. Of the 93 controls, 39 (42%) lived in the lower estate, and 54 (58%) in the upper. The odds ratio for illness after drinking tap water was 8·35 with 95% CI 1·05–66·4 (P=0·045). When the amount of tap water consumed was considered as an ordinal variable with consumption of 1–3 glasses as baseline, consuming 4–6 glasses of water daily gave an odds ratio for illness of 13·6 (95% CI 1·58–116·87, P=0·017) and 7–10 glasses gave an odds ratio of 85 (95% CI 6·46–1118·9, P=0·001). None of the 15 laboratory-confirmed cases had been exposed to cold takeaways, puppies, kittens, or farm animals. No other foods or drinks were associated with illness. As in the cohort study there was an association between home delivery of milk and illness (OR 12, 95% CI 3·5–40·8, P<0·001). However, consumption of milk was not significantly associated with illness (OR 1·29, 95% CI 0·37–4·38, P=0·687). No laboratory-confirmed case that received home-delivered milk had a history of bottle-tops being pecked by birds.
Microbiological investigations
The Laboratory of Enteric Pathogens cultured two serotypes of Campylobacter jejuni (HS23 phage type 1 and HS8) from faecal specimens of cases identified following 4 October.
Environmental investigations
Routine weekly sampling of the dual-chambered upper supply service reservoir from a common outlet by the water company had been satisfactory on 15 September. On 18 September low levels of contamination (10 coliforms and 8 E. coli/100 ml) were detected following heavy rainfall. Free chlorine level had been <0·1 mg/l and total chlorine 0·1 mg/l, below the target level of 0·2 mg/l. The water company had manually dosed the reservoir with sodium hypochlorite and flushed the associated mains before re-sampling the reservoir and distribution system. Sampling on 19–20 September did not reveal any further indicator organisms from the reservoir but free chlorine levels were still low at <0·1 mg/l. A sample from a household tap supplied by the upper estate service reservoir contained 1 faecal coliform/100 ml leading to further mains flushing. The company introduced daily manual sodium hypochlorite dosing of the reservoir on 21 September and reverted to routine weekly sampling. A reservoir sample of 28 September showed no indicator organisms with free and total chlorine 0·1 mg/l. From 30 September the reservoir was sampled daily. The water company reported no documented bacteriological failures prior to 18 September. However, mean free chlorine in the reservoir since May 2000 was <0·1 mg/l.
Figure 3 shows the temporal association between chlorine levels in the upper supply service reservoir and numbers of cases of diarrhoeal illness reported by estate residents. Chlorine levels were rarely above 0·1 mg/l for weeks before the indicator organisms were detected on 18 September. An increase in the frequency of diarrhoeal illness was reported from 11 September. The water company had not tested bacteria or chlorine levels for the period 20–28 September. Faecal streptococci and clostridia were detected on 4 October in the absence of indicator organisms. Despite boosting, chlorine levels had fallen once more to <0·1 mg/l by 21 November.
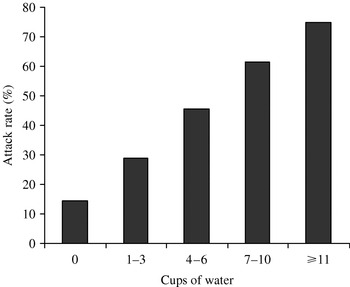
Fig. 2. Association between volume of tap water consumed per day and rate of illness in upper estate residents.

Fig. 3. Temporal association between total chlorine count (–––) in upper estate service tank and cases of diarrhoea (- - -) in estate residents.
Inspection of the upper estate supply service reservoir site externally revealed that the reservoir lay beneath a sheep-grazing field shielded by a protective fence within which was a mature tree. The water company reported that physical external inspection appeared satisfactory. One compartment was internally inspected on 2 October and returned to use following cleaning on 9 October. The full internal inspection of the second reservoir compartment on 11 October revealed a crack in the roof, allowing seepage of surface water through the roof. Rainfall for the month of September was 150% of the monthly average.
DISCUSSION
This is the first documented waterborne outbreak of campylobacteriosis recorded in a chlorinated public water supply in the United Kingdom. Two water-borne outbreaks of campylobacteriosis in the United States were attributed to inadequate chlorination accompanied by heavy rainfall and a fault in the distribution system [Reference Vogt2, Reference Sacks3]. Contaminated water from untreated private water supplies, lakes and streams has been previously identified as a vehicle of both sporadic cases and outbreaks of campylobacteriosis [Reference Furtado6–Reference Palmer9]. Contamination of surface and ground water occurs by run-off from agricultural land used by livestock and is exacerbated by heavy rainfall or melting snow [Reference Melby10–Reference Millson12].
Table 3. Laboratory-confirmed campylobacter enteritis: nested case-control study
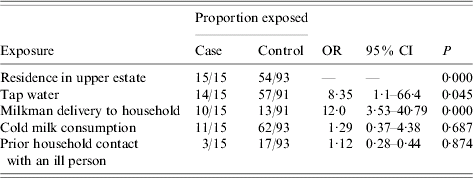
OR, Odds ratio; CI, confidence interval.
This outbreak affected 281 residents of a South Wales housing estate. The demarcation of the estate into two discrete water-supply areas allowed comparisons that confirmed the source. There was a dose-related association between diarrhoeal disease and exposure to upper estate tap water supply.
The cohort investigation provided strong epidemiological evidence of waterborne transmission according to US and UK classifications [Reference Barwick13, Reference Stanwell-Smith14]. The nested case-control study supported the cohort study and strengthened the conclusion that upper estate tap water consumption was responsible for the outbreak.
The epidemic curve for residents of the upper estate suggested a common source outbreak. A mechanism for transmission existed in that the concrete reservoir allowed seepage of surface water contaminated by agricultural waste following heavy rainfall on the surrounding hills. Free chlorine levels in the service reservoir were inadequate to deal with the bacterial load and difficult to sustain. Campylobacters are not routinely tested for by water companies and can be difficult to culture as stressed campylobacters may adopt a viable non-culturable form [Reference Gondrosen15]. Campylobacter was not cultured from water samples. However this does not exclude the possibility that it was present.
Inadequate or failed milk pasteurization has been linked to previous outbreaks [Reference Jones16–Reference Porter and Reid19]. However processes at two independent dairies would need to have failed in this instance. Consumption of home-delivered milk was not a statistically significant risk factor for illness. Having the bottle-tops of home-delivered milk pecked by birds is known to account for some sporadic campylobacter infections [Reference Riordan, Humphrey and Fowles20, Reference Stuart21] but no confirmed case in this outbreak reported pecked bottle-tops.
Only 15 cases of C. jejuni infection were laboratory confirmed. It is likely that many outbreak cases were undiagnosed either because they did not attend their doctor (62·8% of those with illness in this cohort) or because their doctor did not send stool specimens for culture. It is well known that there is generalized underdiagnosis, underreporting and under-identification of infectious gastrointestinal illness. A British study found that one case is reported to national surveillance for every 1·5 laboratory notifications, 3·6 cases in primary care and 8·7 cases of campylobacter infection in the community [Reference Wheeler22].
The findings of this outbreak investigation have implications for the UK water industry, for local authorities and health agencies that may investigate future potential waterborne outbreaks. The authors reported their findings to the Drinking Water Inspectorate (DWI) of England and Wales in June 2001. The DWI reported conclusions and recommendations arising from this outbreak in August 2004 [23]. In its assessment of the incident, the DWI accepted the epidemiological conclusions of the outbreak control team but was very critical of the role of the water company in the outbreak. It concluded that the company did not follow best practice for recognizing and dealing with microbiological incidents and emergencies. In the report the water company was criticized for delayed notification of the incident and for providing reports that contained inadequate and conflicting information. The company's sampling strategy during the early stages of the incident was too limited and was not escalated sufficiently upon detecting faecal coliforms in two spatially and temporally related samples.
It was concluded that the company could have identified which compartment was defective at an earlier stage and could have taken large volume samples from each compartment by dipping, with enhanced analysis for faecal indicator organisms. It was concluded that the company did not seek appropriate or timely expert microbiological advice and did not act to collect and preserve water samples from the distribution system in anticipation that they may later be required for pathogen analysis. In this regard, outbreak control teams investigating possible waterborne outbreaks could consider obtaining advice and field investigation services, at an early stage, from an organization independent of the water company.
The service reservoir was repaired and permanent booster chlorination facilities commissioned on 29 November 2000. Whilst the DWI concluded that the company had supplied water that was not wholesome, it commended the company for rectifying the service reservoir defects once these had come to light. The DWI reported that the water company responded positively to its critical assessment of the incident and took steps to improve in the areas of concern highlighted, including the value to be gained from sharing the lessons learnt widely with public health professionals.
Campylobacters are the commonest bacterial cause of infectious intestinal disease causing considerable morbidity. They are ubiquitous in the animal kingdom. Minimizing transmission is important wherever animals or their products link with human food and water sources. Service reservoirs are common in rural communities and need a programme of regular maintenance and internal inspection. The role of water in the aetiology of sporadic campylobacteriosis may be underestimated.
ACKNOWLEDGEMENTS
Robert Davis, Jennifer Harries, Richard Lewis and John Porter assisted with field work and data entry. We are grateful to the GPs and residents of the affected housing estate for their cooperation in the outbreak investigation. Department of Gastrointestinal Infections, Centre for Infections, Health Protection Agency provided support with microbiological investigations. Welsh Water Dwr Cymru provided the outbreak team with the results of their microbiological and environmental investigations.
DECLARATION OF INTEREST
None.








