INTRODUCTION
Thermotolerant Campylobacter, primarily Campylobacter jejuni and Campylobacter coli, are the most common bacterial causes of human gastrointestinal disease in Denmark [1], EU [2] and many other industrialized countries worldwide [3]. Generally, the increase in human Campylobacter cases started in the 1990s, and in many countries the incidence continues to increase [3]. It is generally accepted that poultry meat is the most important source of foodborne Campylobacter infections [3]. In Denmark, fresh, chilled broiler meat has been identified as a major risk factor of sporadic campylobacteriosis [Reference Wingstrand4].
The first Danish initiatives to combat Campylobacter were initiated in the 1990s, primarily by the poultry industry and the Danish food authorities and comprised hygienic measures at farm level as well as initiation of a monitoring programme of broiler flocks and retail food, especially poultry meat. Moreover, consumer information was also included (Table 1). The initiatives were much inspired by work done in Sweden on Campylobacter colonization of broiler flocks [Reference Berndtson5].
Table 1. The first Danish initiatives to control Campylobacter

In 1998, Campylobacter was included in the national governmental pathogen strategy, which was anchored in the principles of Food Safety Risk Analysis [6, 7] and in 1998 a risk profile for pathogenic Campylobacter spp. in Denmark was prepared [8]. The risk profile recommended continuing the management process commissioning a quantitative risk assessment of Campylobacter in food. Consequently, a quantitative risk assessment of C. jejuni in broiler meat was published in 2001 [Reference Rosenquist9]. C. jejuni in broiler meat was subjected to assessment, as this pathogen–food combination was identified as the major risk for human Campylobacter infection.
Based on the outcome of this risk assessment, national research projects as well as experiences from Iceland [Reference Stern10], a voluntary intervention strategy for Campylobacter in broiler production was developed in collaboration between government, non-governmental organizations and the poultry industry in 2003 (Table 2). The intervention strategy comprised initiatives throughout the production chain; primary production, processing and consumers. One of the key elements of the Danish strategy was to reduce numbers of campylobacter on broiler meat [Reference Jensen11]. This was based on the Danish risk assessment, which predicted that a 2 log10 reduction of the concentration of C. jejuni on the meat could result in a 30-fold decrease in the number of human Campylobacter cases (related to consumption of broiler meat) [Reference Rosenquist9]. To obtain a similar reduction of the number of human cases, the prevalence of broiler flocks should be reduced or kitchen hygiene should be improved 30-fold. Based on the experience at that time, it was assumed that a change of that magnitude of flock prevalence and/or kitchen hygiene would be difficult to obtain, whereas it would be relatively easy to implement the intervention of voluntary freezing of colonized broiler flocks, which had already proven effective as an intervention in Iceland [Reference Stern10, Reference Georgsson12]. Channelling Campylobacter-negative flocks to production of fresh, chilled broiler meat and Campylobacter-positive flocks to frozen products, has also been suggested as a promising intervention strategy by several researchers [Reference Havelaar13–Reference Rosenquist, Boysen and Borck15]. Therefore, it was decided to channel Campylobacter-positive broiler flocks to frozen production, as much as possible, as freezing is known to reduce campylobacter counts by about 2 log10 units [Reference Georgsson12, Reference Sandberg16].
Table 2. Initiatives to control Campylobacter in the Danish voluntary control strategy from 2003

In 2008, a new 5-year action plan against Campylobacter was launched by the Danish Government (Table 3). This was developed in cooperation between the industry, the Danish Veterinary and Food Administration and the Technical University of Denmark and was guided by recommendations from an Expert Consultation held in Copenhagen in 2007 [Reference Rosenquist, Boysen and Borck15]. The action plan aims at reducing the prevalence of Campylobacter in Danish broiler flocks and in Danish broiler meat even further, and innovatively the plan also focuses on reducing the risk of acquiring Campylobacter infections from imported broiler meat.
Table 3. The Danish action plan to control Campylobacter 2008–2012

* Samples collected at wholesale.
In Denmark, testing for Campylobacter in every broiler flock at slaughter has been part of the national monitoring programme since 1998. However, in order to be able to channel the production, in 2003, the two major slaughter companies also initiated a private monitoring programme where all flocks are sampled on farm 7–10 days before slaughter. At retail, broiler meat has been monitored since 1995 and from 2004 onwards samples have also been collected at two large slaughterhouses handling more that 98% of Danish broilers (Table 2). In addition, campylobacteriosis in humans has been notifiable since 1980. In this paper, we present some of the Danish monitoring data on Campylobacter in broilers, broiler meat and humans in order to identify any trends in the occurrence of Campylobacter. The main objective of this paper was to explore if any changes coincide with the implementation of intervention initiatives in the broiler production chain from farm to fork.
MATERIALS AND METHODS
Campylobacter in broiler flocks
Since 1998 all Danish broiler flocks have been tested for Campylobacter at the point of slaughter. The number of flocks tested has varied from about 6000 in 1998 to about 4500 in 2007 [17]. From 1998 to the end of 2001, 10 cloacal swabs were collected at slaughter; these were pooled and tested using conventional microbiological techniques as described by Wedderkopp et al. [Reference Wedderkopp18]. From 2002, pooled samples were analysed using the PCR detection method described by Lund et al. [Reference Lund19]. In 2002, the major slaughter companies also initiated testing of flocks on farm. Samples were collected as one pair of sock samples on the farm and analysed using the same PCR method as described for cloacal swabs.
Campylobacter on meat after processing
Since 2004, to evaluate the effect of channelling Campylobacter-positive flocks to frozen production, samples of fresh, chilled broiler meat have been sampled at two Danish slaughterhouses, processing more than 98% of domestic broilers. In the first two years, 18 samples were collected weekly at each slaughterhouse. For economical reasons, the sample size was reduced to 10–12 samples per week in 2006 and 10–12 samples every other week in 2007. Pieces of broiler meat, cut from the surface of the samples, were analysed for numbers of thermotolerant campylobacter according to a quantitative procedure described by Rosenquist et al. [Reference Rosenquist20] based on rinsing of the sample 1:1 (wt/wt) and plating of appropriate 10-fold dilutions onto selective Abeyta–Hunt–Bark (AHB) agar plates with 0·1% triphenyl tetrazolium chloride added for red-staining of colonies. Samples were categorized as negative if campylobacter were not detected (detection limit: <10 c.f.u./g). Conversely, samples were categorized as positive whenever campylobacter were detected.
Campylobacter in human gastrointestinal disease
Data on human infections were obtained from the Danish laboratory surveillance system. Clinical microbiology laboratories (of which there are 14 in Denmark, all public) are required to report all first-positive (i.e. the first diagnosis for any individual within a 6-month period) cases of campylobacteriosis to Statens Serum Institut every week. Foreign and domestically acquired infections can only in part be separated as only a minor subset of notifications include information about the country of infection. Data are entered into a national database, the Register of Enteric Infections. Although techniques may vary between laboratories, faecal samples from patients suffering from diarrhoea were generally analysed by emulsifying a standardized inoculum in sterile saline and culturing on modified charcoal cefoperazone deoxycholate agar (mCCDA), as described by Engberg et al. [Reference Engberg21].
Statistics
Analysis of trend was carried out using linear regression analysis in Microsoft Office Excel 2003 and analysis of quantitative data was done by analysis of variance using the statistical software SAS 9.1.3 (SAS Institute Inc., USA). An α-value of 0·05 was considered to be statistically significant in the statistical analyses.
RESULTS
Campylobacter in broiler flocks
Since the interventions were introduced in broiler production, Denmark has experienced an overall decrease in the prevalence of Campylobacter-positive broiler flocks at slaughter from 43% in 2002 to 27% in 2007. The prevalence of Campylobacter in the broiler flocks at slaughter had a distinct seasonal pattern, with the highest prevalence observed during June–November (up to 78%) and the lowest prevalence observed during December–May (down to 7%) (Fig. 1). For the high-prevalent period the overall prevalence decreased from 55% in 2002 to 39% in 2007. For the low-prevalent period (December–May) the prevalence decreased from 27% in 2002 to 20% in 2003 and has since remained stable at 13–17% (Fig. 2). Thus, for both the high- and the low-prevalent period of the year, Campylobacter prevalence has decreased.
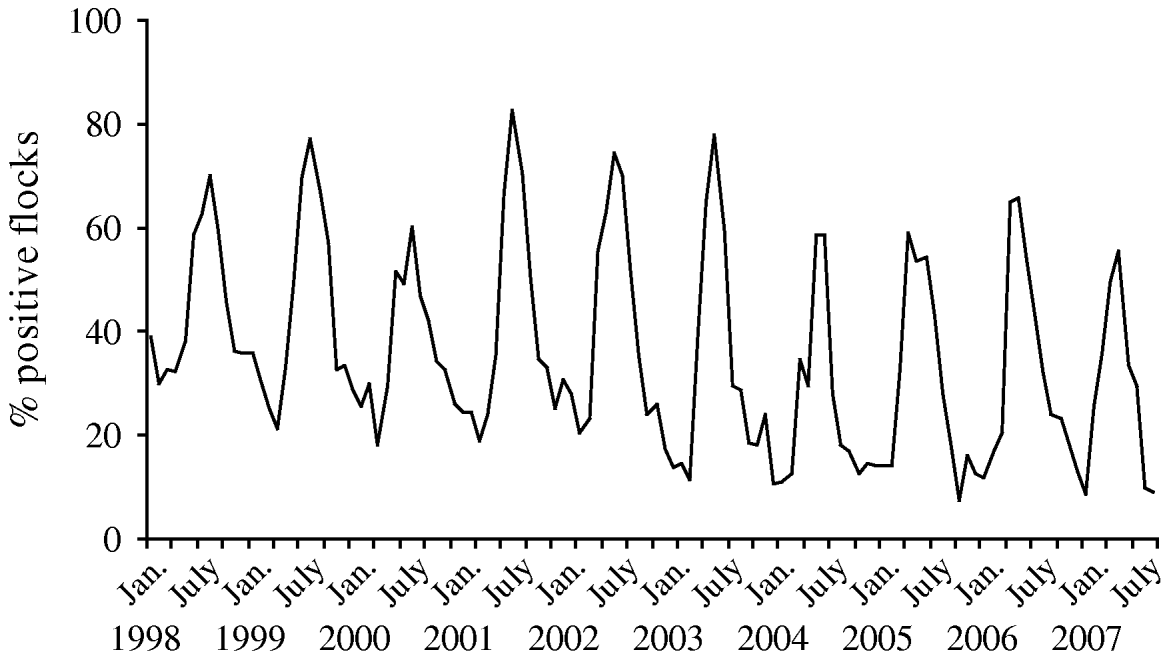
Fig. 1. Prevalence (in percent) of broiler flocks colonized by thermotolerant Campylobacter tested by cloacal swabs at slaughter, by month, 1998–2007.
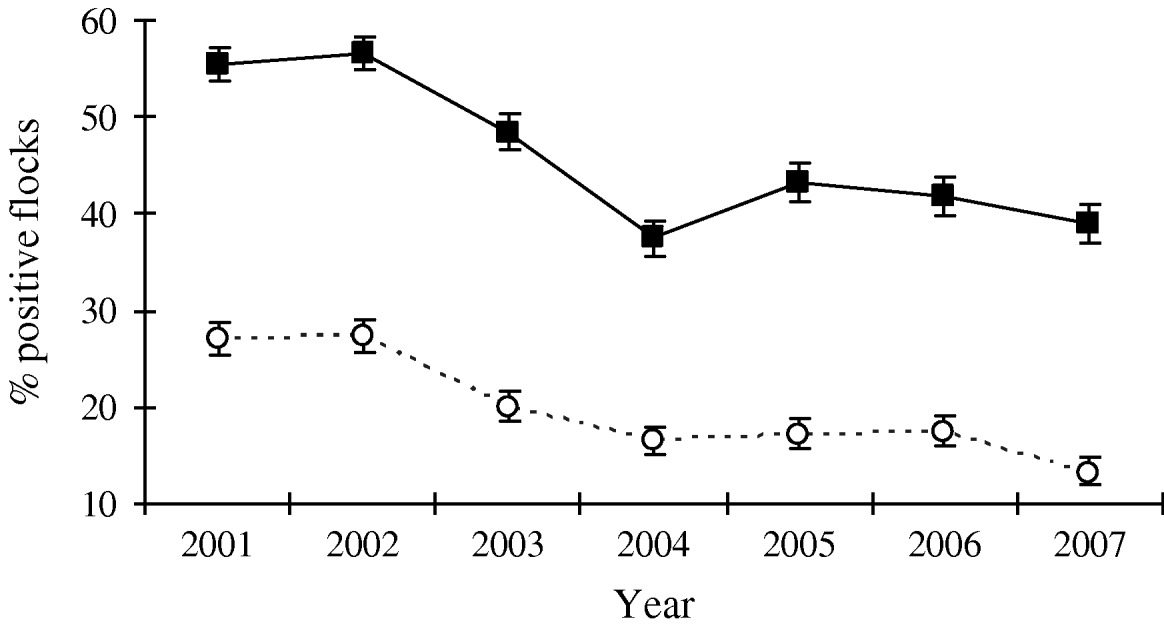
Fig. 2. Prevalence (in percent) of broiler flocks colonized by thermotolerant Campylobacter at slaughter, divided into a high-prevalent period (June–November; –▪–) and a low-prevalent period (December–May; - -○- -), with 95% confidence intervals, 1998–2007. Prevalence estimation is based on the sum of samples in the particular periods (positives and total).
Campylobacter in meat after processing
The percentages of Campylobacter-positive samples of chilled broiler meat collected at two Danish slaughterhouses are presented in Figure 3. The number of Campylobacter-positive samples shows a seasonal pattern similar to that of the Campylobacter prevalence of broiler flocks. On a weekly basis, fewer high peaks of Campylobacter-positive samples were observed in 2005, 2006 and 2007 compared to 2004, indicating a smaller fraction of meat being contaminated. Furthermore, the overall seasonal peak became less pronounced during the monitoring period from 2004 to 2006. In 2004, the total annual percentage of Campylobacter-positive samples was 17·8%. This fell to 12·3% in 2005 and fell further to 7·9% in 2006 and 8·1% in 2007. The decreasing trend from 2004 to 2006 is statistically significant (P=0·042). In 2007, the occurrence was similar to 2006. In regard to numbers of campylobacter on positive broiler meat samples, the mean concentration showed little variation from 2004 to 2007 (P>0·05).
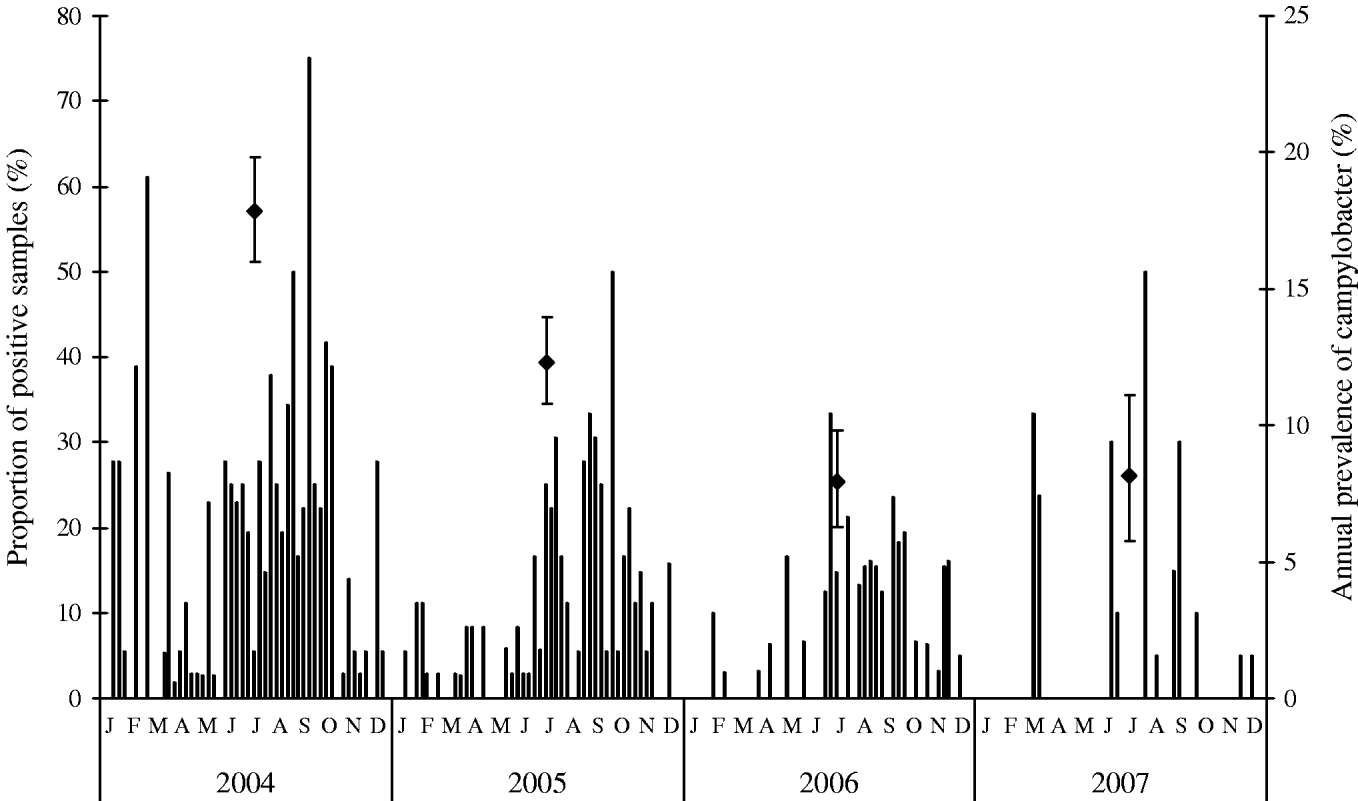
Fig. 3. Weekly mean (columns) and annual (◆) prevalence (in percent) of Campylobacter-positive samples of fresh, chilled broiler meat after processing in two Danish slaughterhouses. Data labels indicate 95% confidence intervals for annual prevalence, 2004–2007.
Campylobacter in human gastrointestinal disease
The number of total registered human campylobacteriosis cases in Denmark began to increase in the mid-1990s and peaked at 4620 cases in 2001, corresponding to an incidence of 82·4/100 000 population (Fig. 4). However, in 2002 the number of cases fell and with some fluctuations has continued to fall; in 2007 a total of 3865 cases were registered, corresponding to an incidence of 70·6. Overall this represents a fall of 12% from 2002 to 2007.
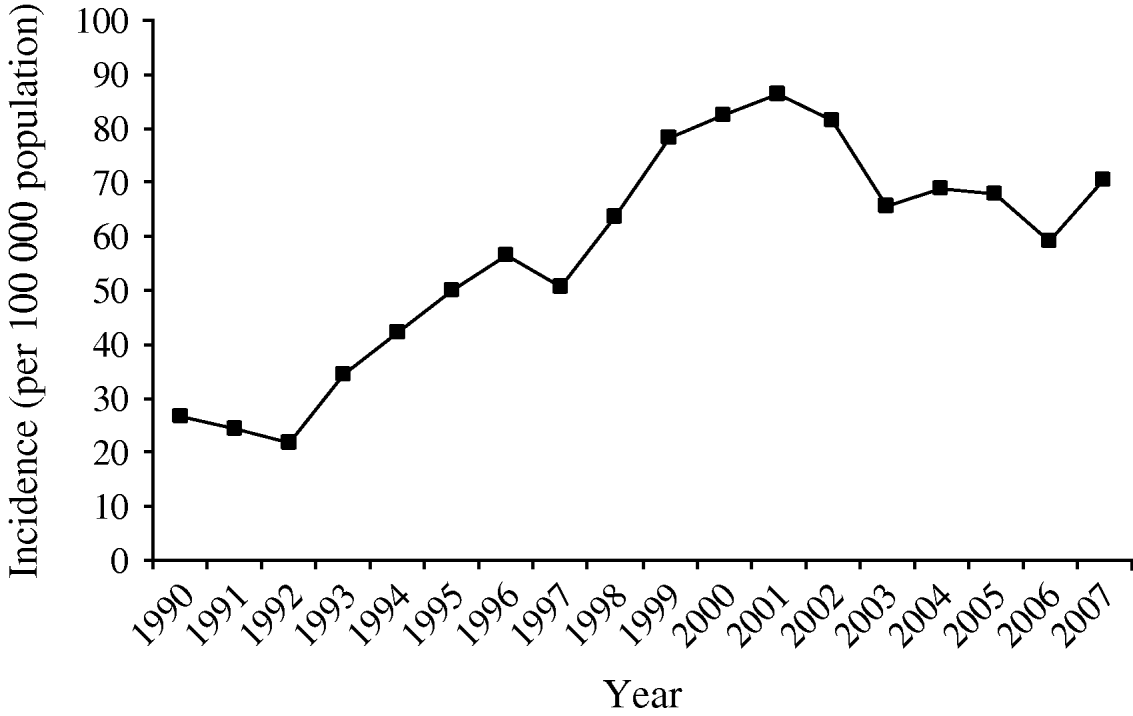
Fig. 4. Incidence of human campylobacteriosis cases in Denmark per 100 000 population, 1990–2006.
DISCUSSION
At farm level, the Danish initiatives to reduce Campylobacter included a number of improvements in biosecurity in and around broiler houses. While it is not possible to identify the effect of each single initiative at the farm, the implementation of the control strategy does coincide with a decrease in the number of positive flocks. Reductions of flock prevalence have also been observed in Sweden, Norway and Iceland, following the implementation of Campylobacter control strategies, in which biosecurity has also played a major role [1, Reference Hansson22, Reference Hofshagen and Kruse23].
The Danish monitoring of the two major broiler meat producers indicated that the voluntary channelling in these slaughterhouses seemed to improve from 2004 to 2006 due to smaller seasonal fluctuations within years and decreasing Campylobacter prevalence for fresh, chilled broiler meat. However, these observations, are probably not only influenced by channelling but also by other voluntary initiatives in the slaughterhouses, e.g. logistic slaughter (the slaughter of negative birds before positive ones) and the implementation of crust-freezing of broiler meat portions. Crust-freezing results in a reduction in numbers of campylobacter of ~0·5 log10 c.f.u. [Reference Boysen and Rosenquist24], which means that meat containing very low numbers will probably test negative after treatment resulting in a reduction in the overall prevalence. Moreover, logistic slaughter is expected to reduce the overall prevalence, but in regard to reduction in numbers of human cases, logistic slaughter is, by several risk assessments, estimated to have only a negligible effect due to the expected low numbers of campylobacter on the originally negative, but cross-contaminated broiler meat [Reference Nauta25].
As previously mentioned, the percentage of meat contaminated with Campylobacter decreased from 2004 to 2006 in the two slaughterhouses investigated, although the mean concentration of Campylobacter on the positive samples remained unchanged. This does not necessarily reflect that a reduction in the concentration of Campylobacter on the meat has not been achieved. Based on the fact that more meat tested negative from 2004 to 2006 in combination with implementation of crust-freezing and logistic slaughter in the slaughterhouses, it is probable that the concentration has been reduced in a proportion of the broiler meat, resulting in the meat testing negative after slaughter.
The observed decrease in the prevalence of broiler flocks and fresh, chilled broiler meat has not translated directly into a considerable drop in the number of human cases. This may be explained by the actual effect being counterbalanced by several factors. First, the broiler meat available at retail in Denmark is not exclusively Danish and nationwide monitoring has shown that Campylobacter is found more frequently in imported broiler meat compared to domestically produced broiler meat [17]. Second, the market share of imported broiler meat has increased from about 20% in 2002 to 40% in 2006 [26, 27]. Third, chilled broiler meat from smaller Danish slaughterhouses is also available at retail. The Campylobacter status of meat from these slaughterhouses is unknown, but will be monitored in future as part of the new action plan. Finally, since the exact proportion of human infections that may be attributed to broiler meat is unknown, there is some uncertainty as to the actual reduction in human cases that may be obtained from controlling Campylobacter in broiler meat. However, it has been suggested that 20–25% of the human cases acquired in Denmark can be attributed to fresh, chilled broiler meat [Reference Wingstrand4].
The first Danish risk assessment from 2001 [Reference Rosenquist9] included only the Danish production of broiler meat. Due to the fact that imported broiler meat also contributes to human exposure to Campylobacter in Denmark, the risk assessment is at the point of being updated to also include imported broiler meat.
In order to bring the number of human cases down further, novel initiatives were included in the Danish action plan from 2008. Currently, flocks are scheduled for channelling based on the Campylobacter status determined by the companies' own sampling and analysis of sock samples using PCR. These samples are collected 7–10 days prior to slaughter. Consequently, there is a risk that negative flocks may become colonized before slaughter and proceed to production of chilled products. The channelling approach could be optimized by using rapid detection methods with similar or increased sensitivity and specificity, allowing for sampling closer to the time of slaughter [Reference Katsma28]. Development of new rapid detection methods was therefore included in the action plan.
In Denmark, as in other countries, a strong seasonality is observed for Campylobacter prevalence in broiler flocks [Reference Hansson22, Reference Meldrum29, Reference Peters30]. This complicates flock channelling. During the high-prevalent period (June–November), fewer Campylobacter-negative flocks are available for chilled meat production and due to logistic difficulties it is not feasible to satisfy the consumer demand using only Campylobacter-negative flocks. The option of freezing all domestically produced, contaminated meat is not feasible, because this would cause a shortage of chilled products during periods, where Campylobacter prevalence in broilers is high. This in turn might lead to increased imports of chilled broiler meat in order to sufficiently satisfy consumer demand. Based on the historical data on Campylobacter in imported broiler meat, this might lead to more contaminated meat on the market, and consequently more human infections. Hence, there is a need to look into control measures other than channelling that could be applied, especially during the high prevalent period. Examples of control measures that were described in the action plan were fly screens, which have proven very effective in preventing introduction of Campylobacter in broiler houses under Danish conditions [Reference Hald, Sommer and Skovgard31] and steam ultrasound treatment of broiler carcases [Reference Boysen and Rosenquist24].
Even though Denmark carries out intensive monitoring of broilers and broiler meat and has a notification system for human campylobacteriosis, it has been difficult to evaluate the impact of the implemented control strategies in general, and on human health in particular, because several factors influence the number of Campylobacter cases. First, broilers are not the only source of human infections, and therefore cases would occur even if Campylobacter was eradicated from the broiler industry. Second, several strategies are voluntary, leaving the level of compliance uncertain. Finally, national control strategies mainly affect broilers and broiler meat produced domestically, while import of broiler meat will inevitably affect the overall Campylobacter status of retail broiler meat within a country. This is why the new Danish action plan also includes initiatives directed against imported broiler meat, e.g. case-by-case based surveillance [1], and the request to retailers and wholesalers to enforce stricter requirements for food safety from their suppliers.
Across Europe other countries have also implemented strategies to control Campylobacter, in particular countries in Northern Europe [2]. The interventions that have generally been implemented are elements of biosecurity, improved hygienic practices at slaughter and consumer education. Similar to Danish observations, countries with a strategy to control Campylobacter in broilers (e.g. Sweden and Norway) have seen a reduction in the prevalence of Campylobacter in broiler flocks and broiler meat, but not a significant drop in the number of human campylobacteriosis cases; however, there have been indications of decreasing trends [2, Reference Hansson22, Reference Hofshagen and Kruse23]. In these countries, the actual effect might also have been counterbalanced by other factors. The only country that has clearly experienced a dramatic decrease in human cases of campylobacteriosis following implementation of control measures in broiler production is Iceland. Since strict control measures were implemented along the whole food chain (birds to humans) in 2000, campylobacteriosis cases fell from 116 cases/100 000 population to <10 cases/100 000 population [Reference Stern10, Reference Callicott32]. The control measures comprised biosecurity at farm, freezing of meat from Campylobacter-positive flocks and intensive consumer education campaigns. One of the major differences between Iceland and other Northern European countries is that only domestic broiler meat is consumed in Iceland. This makes national control initiatives much more effective.
In conclusion, the implemented strategy to control Campylobacter in Denmark has had a positive effect; reducing the prevalence in broiler flocks and broiler meat. A small decrease in the number of human cases has also been observed. The explanation for the effect on human cases not being more significant is probably due to other factors counterbalancing the effect of the implemented interventions. In particular, imported broiler meat and other sources of infection than broilers are assumed to have influenced the limited effect on humans. To combat Campylobacter it is important to focus not only on domestic production, but also on imported products and therefore shared activities among broiler-producing countries are essential.
ACKNOWLEDGEMENTS
The authors acknowledge the laboratory personnel at the respective institutions for the immense work with microbiological analyses.
DECLARATION OF INTEREST
None.









