Foot-and-mouth disease (FMD) is a highly contagious disease affecting domestic and wild cloven-hooved animals (Artiodactyla). It remains the single most difficult animal viral disease to control and causes severe economic losses to the livestock industry [Reference Alexandersen and Mowat1, Reference Alexandersen2].
Camelids belong to the suborder Tylopoda, order Artiodactyla [Reference Wernery and Kaaden3]. Although they regurgitate and re-chew their food like ruminants, camels were divided from Ruminantia (cattle, goats, sheep, deer, etc.) around 40 million years ago [Reference Fowler4]. The present camels reared in areas of the Old World (tribe Camelina) are Bactrian camels (Camelus bactrianus) and dromedary camels (Camelus dromedarius). Around 18 million one-humped camels (dromedaries, sometimes referred as the Arabian camel) are bred in North and East Africa and the Middle and Far East. On the other hand, two-humped Bactrian camels (around 1·4 millions) are found especially in areas with rigorous, cold climate such as China, Mongolia, Turkmenistan and the former USSR (FAOSTAT, 2004). Since camels inhabit geographic areas which are also endemic for FMD, it is of great concern whether they may serve as FMDV reservoirs and potential carriers. The present knowledge on FMD in camelids was recently reviewed by Wernery & Kaaden [Reference Wernery and Kaaden5]. All observations on natural and experimental FMD, including our own studies in dromedaries failed to convincingly show that these animals have the same susceptibility to FMDV as ruminants or pigs (S. Alexandersen et al., unpublished data) [Reference Wernery and Kaaden5, Reference Wernery6]. Very little is known about the susceptibility of Bactrian camels to FMDV infection. Attempts to extrapolate our findings of low susceptibility to FMDV infection observed in dromedaries to all camel species may lead to false conclusions. Here, we give strong arguments not to do so. Early investigations described FMD in Bactrians in Kazakhstan and Russia as affecting their lips, buccal mucosa and feet [Reference Wernery and Kaaden5]. Other researchers suggested that FMD-like clinical signs were mistaken for camelpox or vesicular stomatitis outbreaks [Reference Wernery and Kaaden5]. Several authors described FMD outbreaks in Mongolia in the 1970s, and more recently in 2001 as affecting Bactrian camels reared together with diseased cattle, goats and sheep, although no samples from camels were tested and the diagnosis was done only on clinical observation (V. Kouba, personal communication to M.L.) [Reference Kouba7].
The virus used in this study was FMDV A SAU 22/92 from the suspension of bovine vesicular epithelium, kindly provided by Nigel Ferris from the World Reference Laboratory for FMD in Pirbright, UK. The titre of the virus was 107·6 TCID50/ml as assayed in primary bovine thyroid cells (BTY). Two adult male dromedaries weighing 400–450 kg, six young male dromedaries weighing up to 200 kg and two mature Bactrian camels [Bactrian camel inoculated (BCI): bull B3 and cow B2] weighing around 500–600 kg which were all serologically negative for FMDV, were inoculated subepidermolingually with 107·0 TCID50 of FMDV type A SAU 22/92 inoculum in a volume of 0·25 ml each [Reference Wernery6]. The inoculated dromedaries were kept in outdoor pens at the Central Veterinary Research Laboratory in Dubai, together with five other naive young male dromedaries which served as direct contact animals. Additionally, four local seronegative sheep were kept in a separated corner of the dromedary camel pen as contact animals from day 1 of the inoculation until day 14 when all sheep were euthanized. The two Bactrian camels were kept in direct contact with dromedary camels in an adjacent pen. Two other local sheep of around 30 kg (S7984, S7955) previously vaccinated twice against FMDV with a trivalent vaccine with aluminium hydroxide adjuvant (Aftovax, Merial, antigens of type O, A and Asia 1) at 6-month intervals (last vaccination administered around 6 months prior to inoculation) were challenged by intradermal inoculation into the coronary band of the left forefoot with the same dose of FMDV type A SAU 22/92 as the camels. Vaccinated sheep as partially immune animals were used to avoid severe clinical disease. These animals were kept as positive FMD controls in an indoor infection unit, separate from the other animals.
All animals were monitored for clinical signs of FMD and rectal temperatures were recorded daily until 7 days post-inoculation (p.i.) and subsequently on days 10, 14, 21 and 28 p.i. Blood and mouth-swab samples were taken from all animals before the start of the experiment and daily until day 7 p.i. and then on days 10, 14, 21 and 28 p.i. and immediately frozen at −80°C until tested. Probang samples were collected using a probang cup on days 3, 7, 10, 14, 21 and 28 p.i. [Reference Wernery6]. Each sample was assayed in BTY cells to determine the presence of infectious FMDV. For viral RNA quantification, TaqMan real-time RT–PCR was preformed on all samples as described elsewhere [Reference Alexandersen8]. Additionally, the sequence analysis of several overlapping PCR fragments corresponding to the VP4–VP1 coding regions of some of the FMDV isolates was performed using the BioEdit platform based on phylip [Reference Hall9]. Serum samples were titrated and tested by solid-phase blocking ELISA (SPBE) and virus neutralization test (VNT) for antibodies against FMDV [Reference Wernery6]. Additionally, antibodies against non-structural viral proteins were analysed using Ceditest® FMDV-NS (CEDI Diagnostics, Lelystad, The Netherlands) according to the manufacturer's instructions.
The inoculated sheep showed low to moderate clinical signs including anorexia and depression, local swelling and small vesicles on the coronary band at the site of inoculation and lameness on the front feet typical for mild FMD [Reference Alexandersen2]. Infectious FMDV was isolated from the serum of only one sheep (S7984) on day 3 p.i. (103 TCID50/ml). Viral RNA loads in serum were detected by real time RT–PCR in sheep S7984 and S7955 from days 2 to 10 p.i. and on day 1 p.i. respectively (Fig. 1 a, b). The presence of the virus was also observed in probang samples, indicating active replication of FMDV. Furthermore, both of these sheep shed the virus in their mouth swabs for several days after inoculation. In both animals the antibody levels were visibly boosted by FMDV inoculation which was observed as the increase up to 4 log of ELISA and VNT antibodies to FMDV type A from days 7–10 p.i. The antibodies against non-structural proteins of FMDV which indicated presence of replicating FMDV in the ovine tissues were also detected at very high levels. Additionally, from day 7 p.i. both sheep developed low titres of antibodies which cross-reacted with FMDV type O (Manisa) in ELISA.
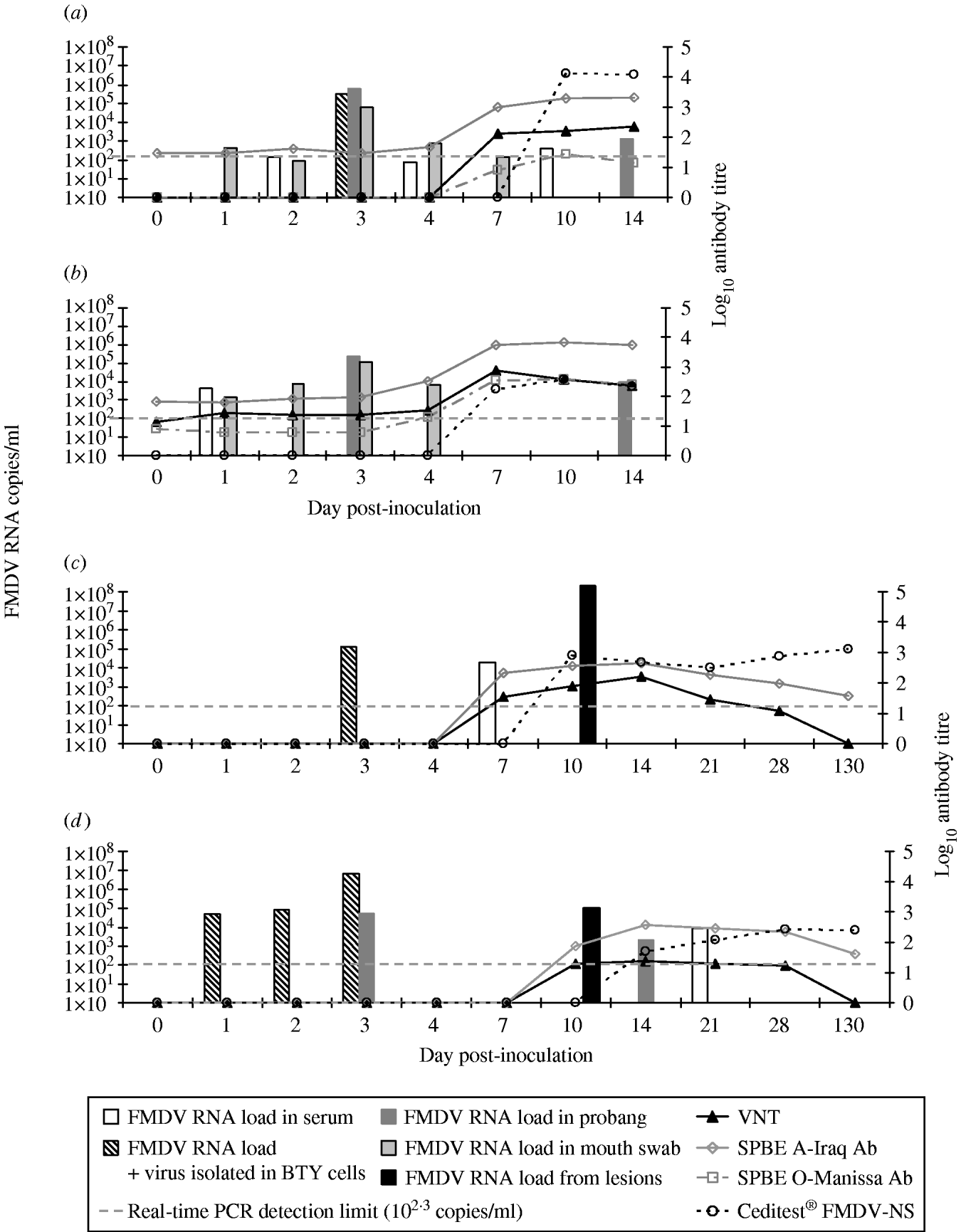
Fig. 1. Timeline of viraemia and seroconversion in the inoculated sheep: S7984 (a) and S7955 (b) and in the Bactrian camels inoculated (BCI): B2 (c) and B3 (d) inoculated with A SAU 22/92. The x-axis is not to timescale.
None of the inoculated and contact dromedaries as well as the contact sheep showed any clinical signs of FMD or developed fever. No infectious FMDV or FMDV RNA was detectable in the sera, probang, or mouth-swab samples from the eight inoculated, five contact dromedaries or from the four contact sheep. None of these camels and contact sheep developed viraemia or developed any antibodies to FMDV as tested by VNT and SPBE ELISA up to 28 days p.i.
Interestingly, a rather pronounced picture of relatively severe FMD was observed in the two inoculated Bactrian camels BCI B2 and B3. Elevated rectal temperatures of 39·0 and 39·2°C were observed in camels B3 and B2 respectively on day 7 p.i. [average mean 37·4°C (s.d.=0·9), n=14]. On the same day, both camels B2 and B3 were off their feed and developed depression (Fig. 2 c) and lameness of the hind feet (Fig. 2 a, b). Later, on day 8 p.i. local inflammation, swelling and exudation on the footpads were observed (Fig. 2 d). The lameness of the hind legs, and the reluctance to walk and stand progressed as the lesions developed further and were most severe on day 10 p.i. Camel B2 lost the entire soles of both hind feet on day 14 p.i. The animals gradually improved and the lesions healed after 21 days p.i. No lesions inside the oral cavity, either on the tongue, hard palate, gingiva, buccal mucosa, lips or pharyngeal region were observed in these Bactrian camels. None of them had any lesions on the front feet or any other skin lesions except the severe lesions on the hind feet (Fig. 2 d).

Fig. 2. Foot-and-mouth disease virus (FMDV) in Bactrian camels. Lameness of hind feet in Bactrian camels B2 (a) and B3 (b); depression (c) and severe lesions caused by FMDV type A SAU 22/92 infection of the hind foot pad of Bactrian camel B2 on day 9 p.i (d) (no lesions observed on the front feet left corner of the picture).
Infectious FMDV was isolated in BTY cells from the serum of Bactrian camel B2 at 3 days p.i. while viral RNA was detected on days 3 and 7 p.i. (Fig. 1 c). However, FMDV was not detected either in the probang samples or in the mouth swabs collected from camel B2 up to 130 days p.i. Viral RNA (108·3 copies/ml) and 105·8 TCID50 of infectious virus were detected in the fluid collected from the lesion on the hind foot from camel B2 on 10 day p.i. This substantiates that these were caused by FMDV infection. Neutralizing antibodies were detected with a mean titre of >1:60 (n=5) from days 7 to 28 p.i. with a peak level of >1:120 at 2 weeks after inoculation (Fig. 1 c). No neutralizing antibodies were found in serum from camel B2 when tested 130 days p.i. The ELISA antibodies against FMDV type A also appeared on day 7 p.i., and the titres was slightly higher compared to the VNT titres and ranged from >1:80 to >1:240 with the peak on day 14 p.i. The ELISA antibodies remained detectable albeit at a relatively low level up to day 130 p.i. (>1:20) (Fig. 1 c).
The Bactrian bull B3 although showing somewhat milder clinical signs of FMD compared with camel B2, had detectable viraemia from days 1 to 3 p.i. and subsequently again on day 21 p.i. (Fig. 1 d). Infectious FMDV was isolated from the serum of camel B3 during the first 3 days and the levels of FMDV corresponded to 104·7, 104·9 and 106·8 copies/ml serum respectively as analysed by real time RT–PCR. No FMDV RNA was detected in mouth-swab samples, but relatively modest amounts of FMDV RNA were detected in probang samples collected at 3 and 14 days p.i. However, no viral RNA was detected in the probang samples collected after 14 days p.i. The load of viral RNA tested in the swab taken from the foot lesion on day 10 p.i. was 105 copies/ml viral RNA, although no viable virus was found in respect to the virus isolation test, probably due to the advanced decay of the tissues as well as detectable circulating neutralizing antibodies (Fig. 1 d). This camel showed detectable antibody titre against FMDV at 10 and 14 days p.i. in both ELISA and VNT. VNT antibodies were detected at low levels with a titre around 1:20 until day 28 p.i. while ELISA antibodies increased to higher titres on days 14–21 p.i. and remained detectable up to day 130 p.i. (>1:40). A correlation between the severity of the lesions caused by FMDV and the antibody titres against non-structural viral proteins was observed. Bactrian B2 had a significantly higher anti-NSP antibody titre than B3 and was first detected on day 10 p.i. (average >1:480, ranging from 1:240 on day 21 p.i. up to 1:1920 on 130 day p.i.) while camel B3 started having detectable anti-NSP antibodies on day 14 p.i. (average >1:120) (Fig. 1 c, d). However, the lower immune response to NSP did not correlate with the more pronounced viraemia in camel B3 compared to B2. No cross-reactivity of the antibodies developed against FMDV type A was detected by SPBE ELISA for type O in any of the Bactrian camel sera tested. Another difference in the response to FMDV in Bactrian camels compared with dromedaries was the development of high titres of antibodies in the Bactrian camels, which lasted up to 130 days p.i. The seroconversion to type A observed in the Bactrian camels was comparable to challenged and previously vaccinated sheep.
Comparison of the nucleotide sequences of the virus collected from the sera on days 1 and 3 p.i. and from one probang sample collected from Bactrian B3 on day 3 p.i. with the full genome sequence of the inoculum FMDV A SAU 22/92 used, showed 100% identity over the 3850 bp analysed. We found two single nucleotide substitutions (in the VP4 and VP2 coding regions) in the sequence of the virus from the foot lesion taken from camel B2 on day 10 p.i. compared to the inoculum virus, however, both of them were synonymous and thus probably simply due to the error-prone viral RNA polymerase.
Presented results demonstrate that dromedary camels are unlikely to be a reservoir of FMDV in the endemic areas nor transmit this virus to susceptible species. While none of the dromedaries were susceptible to FMDV infection, both inoculated Bactrians showed moderate to severe clinical signs of FMD although the onset of FMD was late (8–14 days p.i.) compared to other susceptible animals. Although the animals did not show any lesions at the site of the inoculation, or in or around the mouth, characteristic lesions with accompanying lameness were observed on the hind feet of both Bactrian camels. FMD in Bactrian camels if possible by natural infection may cause a temporal decrease in their productivity and has possibly occurred in Mongolia according to anecdotal information [Reference Kouba7].
The presence of FMDV in probang samples was detected only in one Bactrian camel and was lower in comparison to the viral RNA load in probang and mouth-swab samples from the inoculated sheep. No FMDV shedding from the oral mucosa (mouth swabs) were observed in the mouth swabs from both Bactrian camels. These findings indicate that although the Bactrian camels became acutely infected with FMDV, they did not become long-term carriers. The low quantity and transient appearance of FMDV in the mouth swabs of the Bactrian camels may also explain the lack of transmission to other camels and to the highly susceptible contact sheep. Pharynx and possibly tonsils are known to be a primary site of FMDV infection in ruminants and early FMDV replication appears to take place in the pharyngeal epithelium where the virus can be detected before the onset of viraemia. In contrast, this early replication in the pharyngeal region appears to be at a very low level or does not occur in the Bactrian camels, in which the primary replication of FMDV may have taken place directly in the epithelium of the feet mediated by virus circulating in the blood after inoculation [Reference Alexandersen8], or alternatively, after primary replication in a hitherto unknown internal organ, e.g. the pancreas as observed in FMDV-inoculated adult mice, skeletal muscles as observed in young mice or in the myocardium as described in young, susceptible species [Reference Alexandersen and Mowat1, Reference Gulbahar10].
The results indicated that the two closely related camel species of Bactrian and dromedary camels possess noticeably different susceptibility to FMDV. Bactrian camels can relatively easily be infected with FMDV under experimental conditions as described here, while dromedaries remain resistant to high doses of both FMDV types A and O. Such differences between relatively closely related species were also the case in elephants, for example. The African elephant (Loxodonta africana) is thought to be resistant to FMDV while Asian elephants (Elephas maximus) are susceptible [Reference Alexandersen and Mowat1].
Future investigations should concentrate on possible factors influencing the observed long incubation period of FMD in Bactrian camels, the primary sites of virus infection and the specificity of the cellular receptors responsible for initiation of FMDV infection in this species.
ACKNOWLEDGEMENTS
We thank Dr Ali Ridha, Administrative Director of CVRL for his continuous support, Graham Belsham for useful discussions and Tina Frederiksen, Tina Pedersen, Jane Borch and Jani Christiansen for technical assistance. Research was supported by the Danish Ministry of Family and Consumer Affairs as well as by HH General Sheikh Mohammed Bin Rashid Al Maktoum. We also thank EPIZONE (FP6-2004-Food-3-A) for additional financial support.
DECLARATION OF INTEREST
None.




