Introduction
Scabies is a common and disabling dermatological condition caused by infestation with the mite Sarcoptes scabiei [Reference Chosidow1–Reference Chosidow3]. Designated as a Neglected Tropical Disease by the World Health Organization, it remains an important public health issue in the United Kingdom (UK) [4], especially in institutional settings such as residential or nursing care homes for the elderly (RNC), where outbreaks are typically prolonged, recognition is often delayed and management is highly challenging [Reference Chosidow1, Reference Scheinfeld5, Reference Hewitt, Nalabanda and Cassell6].
Scabies is mainly spread through direct skin to skin contact, and less commonly through fomites. Symptoms tend to present 4–6 weeks after exposure due to a delayed hypersensitivity reaction to the scabies mites' faeces and eggs, or within a week if after repeated infestation [Reference Chosidow3, Reference Green7, Reference Walton8]. The classical presentation is an erythematous papular rash and pruritus, which is typically worse at night [Reference Chosidow3, Reference Hicks and Elston9].
The elderly, young and immunocompromised are particularly vulnerable to scabies [Reference Banerji10, Reference Johnston and Sladden11], and RNC are especially susceptible to institutional outbreaks [Reference Mounsey12]. The typical distribution of clinical signs may differ in the elderly [Reference Walton8, Reference Bouvresse and Chosidow13, Reference Cassell14]; residents may be asymptomatic or have subtle signs, adding to difficulty in diagnosis in this population and contributing to delayed diagnosis. Residents with dementia are at increased risk of scabies [Reference Cassell14]. The highly contagious crusted form of scabies is also difficult to recognise in RNC residents, where it may occur more commonly due to misdiagnosis and treatment with corticosteroids. It presents with a hyperkeratotic rash which may lack the characteristic itch and be more easily transmitted due to high mite burden [Reference Hicks and Elston9, Reference Sunderkotter15].
Management of scabies outbreaks
An outbreak of scabies can be defined as two or more cases of classical scabies, or a single case of crusted scabies, linked by time in the same environment [Reference De Beer16]. The control of outbreaks in RNC is time-consuming and cost-intensive, requiring mass treatment of infected cases and contacts. It is complicated by issues including atypical presentation, close proximity of residents, carers and visitors, mental capacity issues, financial responsibility for treatment and logistical barriers of mass treatment [Reference Sunderkotter15, Reference White17, Reference Stoevesandt18]. Here we focus on outbreaks in RNCs only.
In the UK, existing national guidelines focus on management of individual cases, and none exist for scabies outbreaks in RNC [19]. In England, local Health Protection Teams (HPTs) predominantly provide advice on the control of institutional outbreaks using locally developed guidelines which are highly variable in their recommendations [Reference White17]. No systematic review has been undertaken of the effectiveness of interventions in outbreaks.
Current drug treatments for scabies outbreak management
Drug treatments for scabies include topical acaricides (such as benzyl benzoate, crotamiton, lindane, malathion, sulphur and permethrin) and oral ivermectin, an anti-parasitic. A Cochrane review of scabies treatments recommended topical permethrin, but concluded ivermectin was an effective oral treatment [Reference Strong and Johnstone20]. Current National Institute for Health and Care Excellence (NICE) guidelines for the treatment of individual cases of scabies recommend permethrin 5% cream as first line treatment, and malathion 0.5% aqueous liquid if permethrin is contraindicated [19]. Treatment is applied to the whole body of all household members and their close contacts, even if asymptomatic, left on for 8–24 h and a second application 1 week later is recommended [19]. Oral ivermectin is unlicensed but can be used on a named-person basis [21]. Itch frequently persists following therapy, and may not indicate treatment failure; symptomatic treatment is recommended when needed [19].
Environmental management measures
There is some evidence of indirect scabies transmission through fomites (clothing, bedding, furniture, carpet) [Reference Mellanby22], suggesting environmental management measures including cleaning of RNCs may have a role in preventing transmission. This transmission is thought to be rare with classical scabies but may occur with the crusted form [Reference Chosidow1], though limited due to mites' inability to survive off human skin for long periods [Reference Heukelbach and Feldmeier2, Reference Hicks and Elston9].
NICE guidelines recommend machine washing clothes, towels and bed linen at 50 °C on the day of first treatment and referral of institutional outbreaks to the HPT to advise on infection control measures [19].
How can outbreak outcomes be measured? The need for a framework
Outbreak measures must address the key dimensions described above: timely diagnosis, drug treatment, environmental management and symptomatic care.
Given the diagnostic and management challenges in RNC described above, these must describe all points in the recognition and treatment pathway in order to assess efficacy of interventions, patient experience and how best to measure outbreak duration and delayed recognition or intervention.
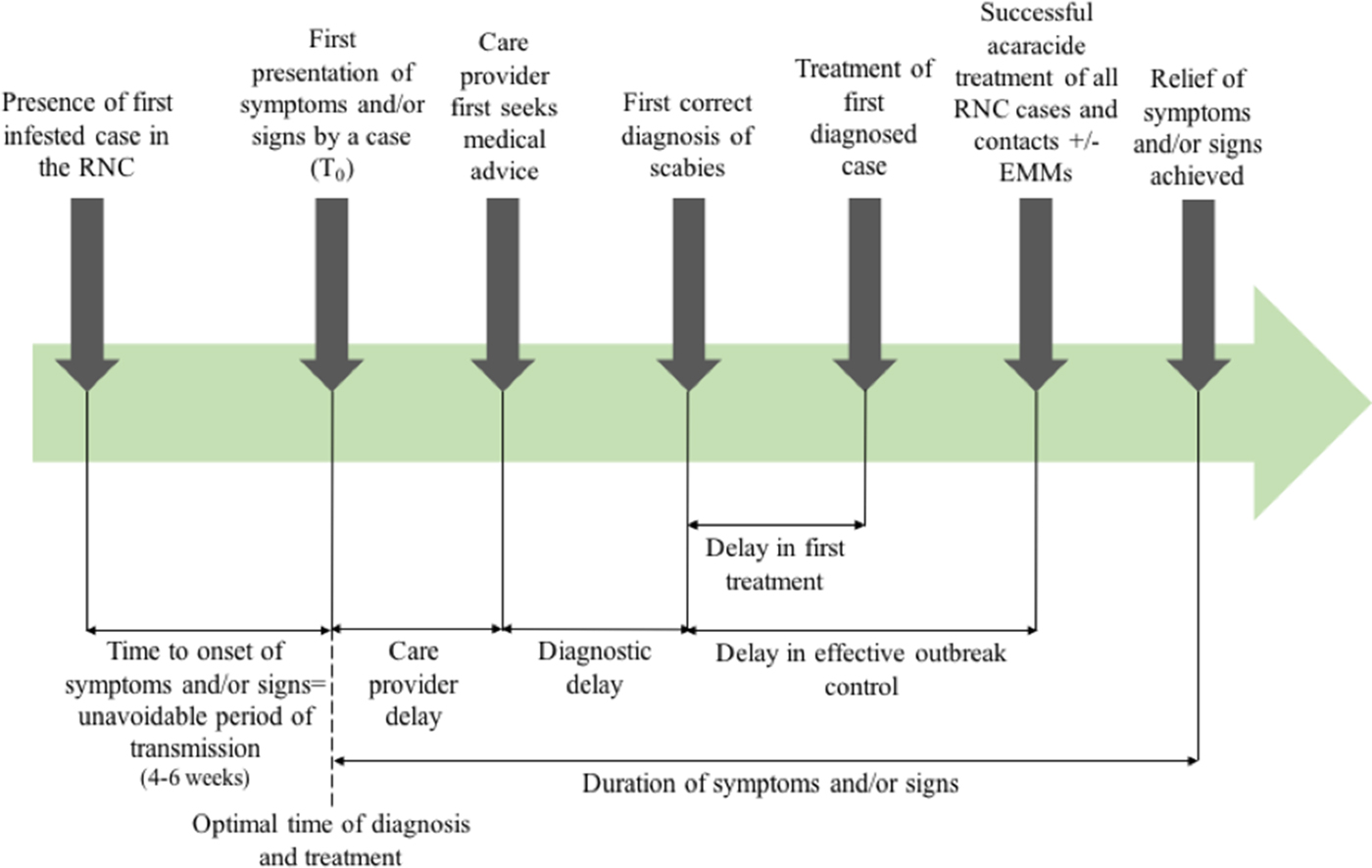
Outcome measures used in previous systematic reviews have included the rate of treatment failure (persistence of original scabies lesions, development of new lesions or identification of mites on a skin scraping), number of new cases following treatment [Reference Strong and Johnstone20], or need for repeat implementation of environmental infection control measures. Figure 1 shows the points at which delay may occur, which will inform this review.
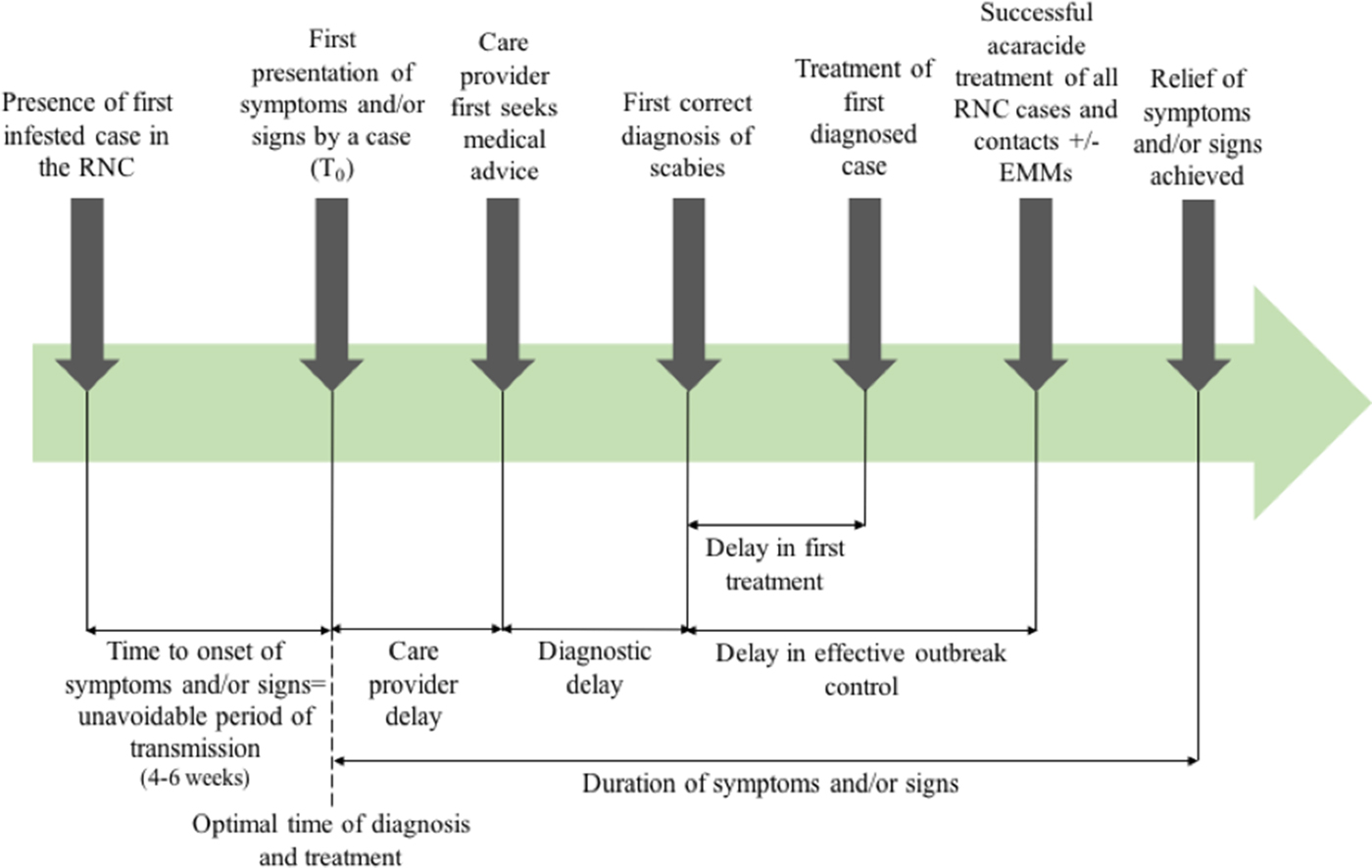
Fig. 1. Outbreak measures in the timeline of a scabies outbreak in a RNC, including points in the diagnosis and management timeline where delay may occur.
In order to provide high-quality care, patients' perception of their care must be considered. Patient-Reported Outcome Measures (PROMs) assess the efficacy of a treatment from the patients' perspective. PROMs in scabies infestation and management may include duration or severity of itch, quality of sleep and rate of secondary infection. Patient-Reported Experience Measures (PREMs) evaluate the patients' views of their experience while being treated [Reference Kingsley and Patel23].
We undertook a systematic review of evidence on the effectiveness of interventions for the management of scabies outbreaks in RNC.
Aims
To systematically review evidence and determine how to best manage scabies outbreaks in RNC. We address two empirical research questions:
1. What is the most effective drug treatment for scabies outbreaks in residential or nursing care homes for the elderly?
2. Which environmental infection control measures should be undertaken to prevent further transmission and prolonged infestation following an outbreak in this setting?
To inform future research, we also address methodological research questions:
1. What are the most useful outcome measures in assessing the effectiveness of scabies management measures?
2. How should delayed diagnosis and treatment be measured?
For the purposes of the current review, ‘elderly’ is defined as a mean age of residents of over 65 years, where described.
Materials and methods
Search strategy
A literature search of four databases (PubMed, Cinahl, Embase and Web of Science) was performed on 7 January 2017 and repeated on 19 July 2017, using the terms ‘(((scabies OR crusted scabies OR sarcoptes scabiei OR scabies mites)) AND (residential home OR care home OR residential facility OR long term care facility OR nursing home)) AND (treatment OR benzyl benzoate OR permethrin OR ivermectin OR malathion OR lindane OR sulfur OR scabicide lotion OR infection control OR washing OR vacuum OR hoover OR cleaning OR carpet OR upholstery OR bedding OR clothes OR isolation OR gloves OR aprons OR care home closure)’.
Citations were retrieved from inception to the date of search. Additional papers were identified using Google Scholar citation searching and PubMed related articles. The PRISMA statement was followed [Reference Moher24]. The search was carried out by two separate reviewers (EJM and JEC). Search results were imported and stored in EndNote Web, duplicates were removed and articles that were not relevant, judged by titles and abstracts, were excluded. All study designs were eligible for inclusion.
Inclusion and exclusion criteria
Articles were eligible for inclusion if they met two criteria: (i) studies of scabies outbreak management in RNC, and (ii) studies describing either drug treatment or environmental infection control measures implemented. Non-English language, animal studies and those describing a scabies outbreak in other settings, were excluded. Both reviewers screened search results for compliance with the inclusion and exclusion criteria; disagreements were discussed and resolved by consensus.
Quality assessment
All studies to be included in the review were assessed for quality by the first author. We intended to assess the quality of evidence and overall strength of recommendations using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations Working Group) criteria [Reference Guyatt25]. However, GRADE differentiates poorly between the quality of non-comparative observational studies, classifying them all as low or very low quality evidence, so that any recommendations for the management of scabies outbreaks in RNC would be weak based on the reports identified (see the ‘Results’ section).
A quality assessment tool was therefore developed from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist [Reference Vandenbroucke26], to capture the quality of observational data for this review. A suite of 17 outcome measures considered suitable for these outbreaks was developed based on this in the absence of a standard description.
Data extraction
Data items extracted included details of the population and setting, number of cases identified, drug treatments and environmental infection control measures implemented and outcome measures reported.
Primary outcome measures were treatment failure of a confirmed case or need for repeat environmental measure implementation. The secondary outcome measure was duration of outbreak (defined as time from identification of infestation of the index case to successful treatment resulting in no new or repeat cases). Adverse events were considered serious if life threating or resulted in death or hospitalisation. Side effects including those which required discontinuation of treatment or caused patient discomfort or dissatisfaction were recorded.
The timescale of the outbreaks including date of first presentation of symptoms or signs by a case, first correct diagnosis of scabies, treatment of first diagnosed case and successful treatment of all RNC cases were extracted where available in order to describe duration of any delays in outbreak management.
Results
Literature search
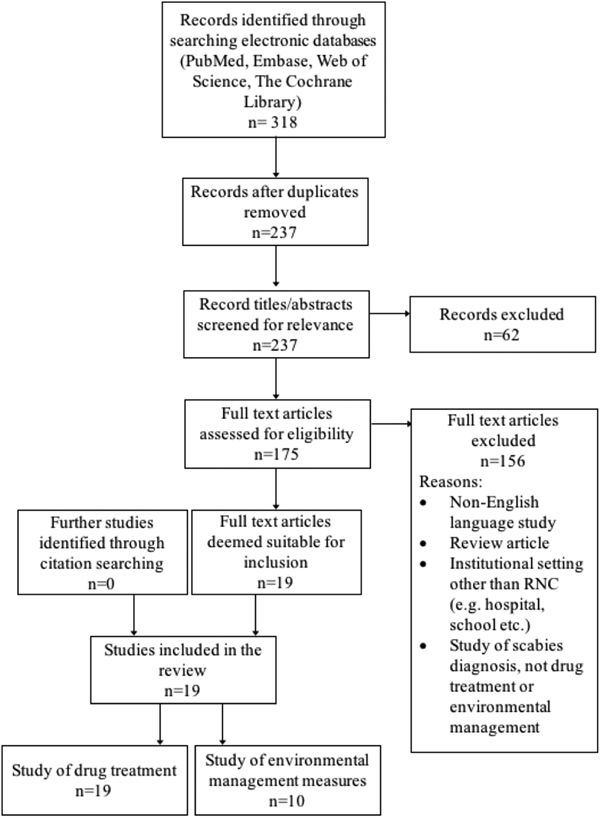
A summary of the literature search is shown in Figure 2. Nineteen studies were included in the review, dating back to 1983. All evaluated the drug treatment of scabies in RNC, and 10 also assessed one or more environmental management measure. The included studies are detailed in Table 1. Nearly all were excluded due to not presenting primary data on an outbreak. These ranged from discussion pieces, editorials and educational articles. A few reported outbreaks in settings that did not meet our inclusion criteria.
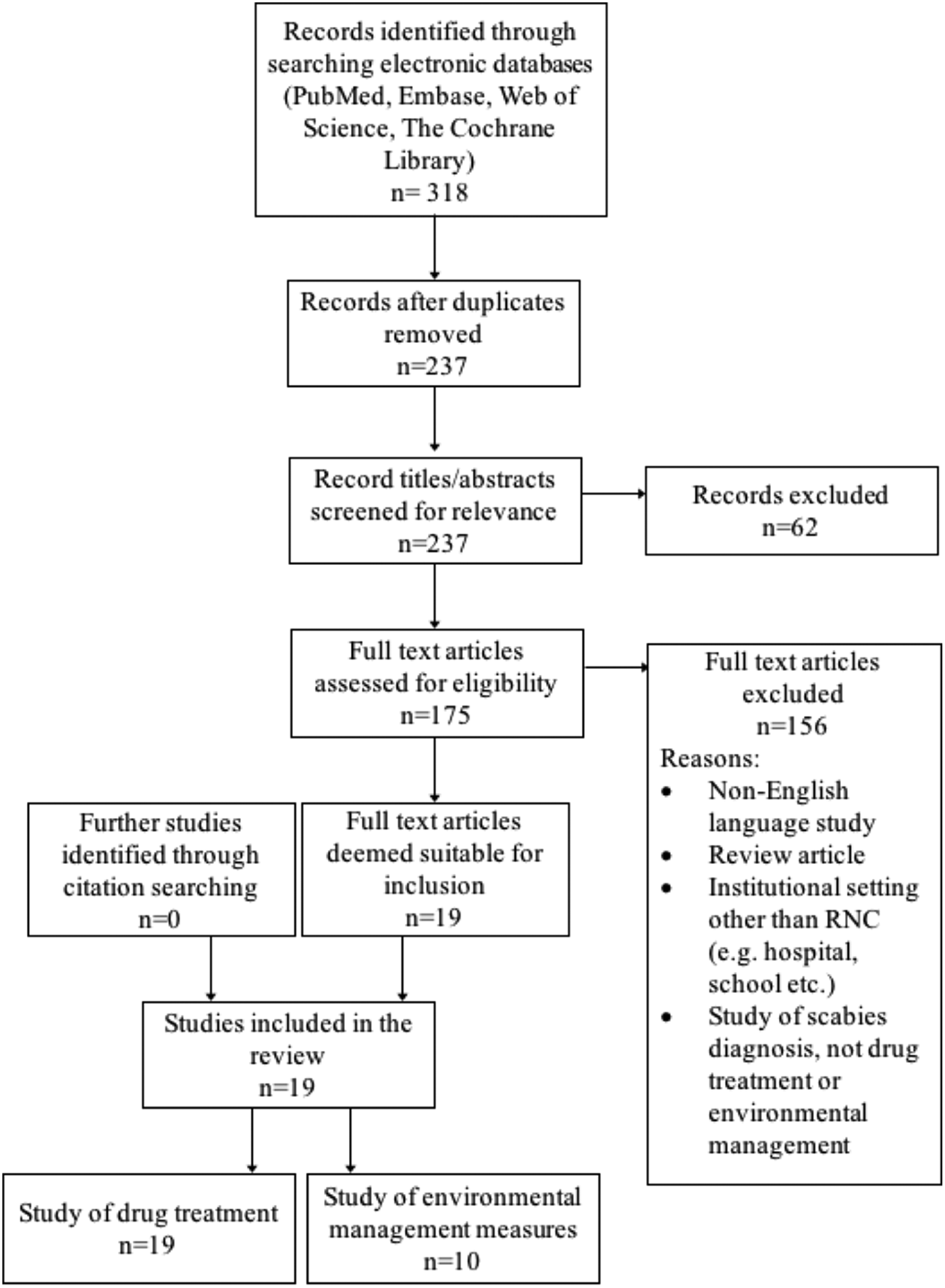
Fig. 2. Flow of study selection at each stage from identification to final inclusion, including study numbers for both drug treatment and environmental management measures.
Table 1. Summary of all studies included in the review, including outcome measures described in reports

Quality assessment
Table 2 shows results of our assessment of included studies using the STROBE derived quality assessment tool. Delays in management and outbreak duration were identified through reporting of dates of outbreak identification, management measure implementation and outbreak conclusion. No case report described all 17 points, and seven reports were particularly poor [Reference Apap, Piscopo and Boffa28, Reference Barkwell and Shields29, Reference Chan31, Reference Hetland33, Reference Papini, Maccheroni and Bruni38, Reference Parish39, Reference Wilson, Philpott and Breer43], detailing less than 10 points.
Table 2. Results of quality assessment tool adapted from the STROBE checklist for observational studies
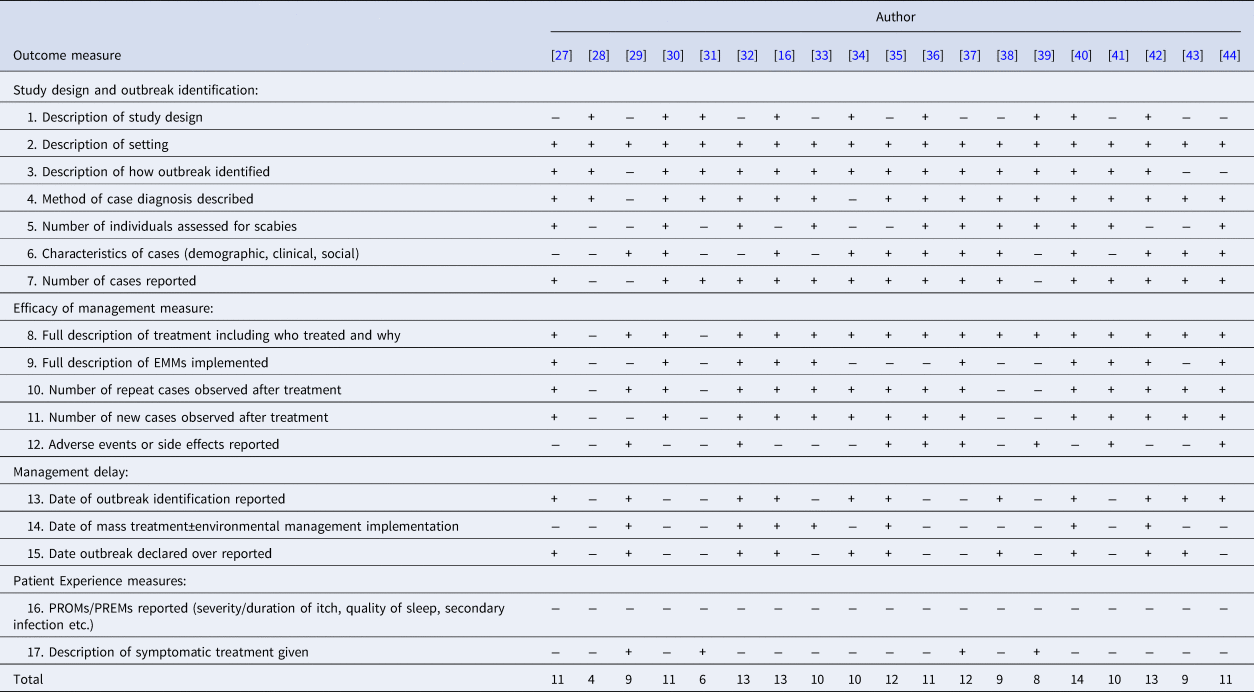
+ Represents criteria achieved. − Represents criteria not achieved.
Outcome measures
The reporting of outcome measures varied markedly. Two case reports [Reference Apap, Piscopo and Boffa28, Reference Chan31] only discussed treatment of a single index case after hospital admission for treatment and did not further describe the outbreak. Five reports did not describe the rate of treatment failure [Reference Apap, Piscopo and Boffa28, Reference Barkwell and Shields29, Reference Chan31, Reference Papini, Maccheroni and Bruni38, Reference Parish39], and three did not report the number of new cases diagnosed after treatment [Reference Apap, Piscopo and Boffa28, Reference Barkwell and Shields29, Reference Chan31]. No definitions of treatment failure were given. Seven articles did not report outbreak duration [Reference Apap, Piscopo and Boffa28, Reference Burns30, Reference Chan31, Reference Hetland33, Reference Moberg, Lowhagen and Hersle36, Reference Paasch and Haustein37, Reference Yonkosky44]. Notably, no PREMs or PROMs were reported in any outbreak.
Outbreak management delays
Delay in diagnosis or in successful treatment was described in all but one case report in which the time scale of the outbreak was not specified [Reference Papini, Maccheroni and Bruni38]. Twelve described a delay in diagnosis [Reference De Beer16, Reference Andersen27, Reference Apap, Piscopo and Boffa28, Reference Burns30, Reference Chan31, Reference Hetland33, Reference Ladbury34, Reference Moberg, Lowhagen and Hersle36, Reference Paasch and Haustein37, Reference Paules, Levisohn and Heffron40, Reference Van den Hoek42, Reference Wilson, Philpott and Breer43], either stating there was a delay or reporting the duration of time cases had symptoms before a diagnosis of scabies was made. Fifteen reported a delay in effective treatment [Reference De Beer16, Reference Andersen27, Reference Barkwell and Shields29, Reference Burns30, Reference Dannaoui32, Reference Hetland33, Reference Millership, Readman and Bracebridge35–Reference Paasch and Haustein37, Reference Parish39–Reference Yonkosky44], due to treatment failures or new cases following treatment. In eight studies [Reference De Beer16, Reference Andersen27, Reference Apap, Piscopo and Boffa28, Reference Chan31, Reference Hetland33, Reference Moberg, Lowhagen and Hersle36, Reference Parish39, Reference Paules, Levisohn and Heffron40], misdiagnosis was reported. Though not all articles specified the duration of delay; where reported, the median duration from onset of symptoms to diagnosis was three months, and median delay in effective treatment was 9 months.
Drug treatments
Over 800 cases of scabies were described in 19 studies. Drug treatment of cases and prophylaxis of contacts (asymptomatic residents, staff and family) varied widely, as did success. Common drug regimens were either topical treatment alone, topical treatment in combination with oral ivermectin, or ivermectin alone. A summary is provided in Table 3.
Table 3. Summary of drug treatments implemented in reports. Alternative treatments used due to new cases arising following treatment or unresolved cases. Note some RNCs reported the use of different drug treatments for different residences affected
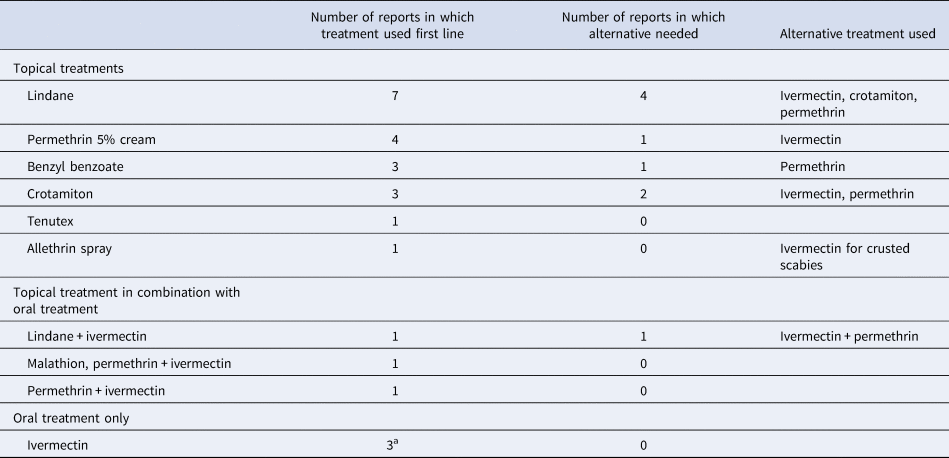
Tenutex = DDT 0.5%, disulphiram 2%, benzyl benzoate 22.5%; commercial purified form of GBHC (gamma benzene hexachloride) is lindane, therefore results combined.
a Staff and contacts were treated with topical permethrin in all three reports.
Permethrin cream was used first in four reports [Reference De Beer16, Reference Paasch and Haustein37, Reference Papini, Maccheroni and Bruni38, Reference Wilson, Philpott and Breer43], which resulted in two repeat cases in one report and three repeat cases in another. These resolved with further permethrin application or oral ivermectin therapy. In two studies other topical treatments were used initially (Lindane, benzyl benzoate and crotamiton) with high levels of treatment failure, before permethrin was used, resulting in resolution of the outbreak [Reference Paules, Levisohn and Heffron40, Reference Yonkosky44]. Of the 14 case reports where topical treatments were used alone, oral ivermectin was required in three for outbreak resolution [Reference Barkwell and Shields29, Reference Dannaoui32, Reference Wilson, Philpott and Breer43]. In two case reports [Reference Paasch and Haustein37, Reference Papini, Maccheroni and Bruni38], crusted scabies cases were identified and treated differently from typical scabies, commonly with a combination of topical treatment and oral ivermectin, which successfully ended the outbreak. Notably, in all case reports in which oral ivermectin was used, it resulted in resolution of the outbreak [Reference Apap, Piscopo and Boffa28, Reference Barkwell and Shields29, Reference Dannaoui32, Reference Ladbury34, Reference Millership, Readman and Bracebridge35, Reference Sullivan, Watt and Barker41–Reference Wilson, Philpott and Breer43].
In 13 case reports [Reference De Beer16, Reference Apap, Piscopo and Boffa28, Reference Burns30, Reference Dannaoui32–Reference Paasch and Haustein37, Reference Parish39, Reference Paules, Levisohn and Heffron40, Reference Van den Hoek42, Reference Yonkosky44], all residents, staff and visiting contacts were treated. The rest varied between residents only [Reference Barkwell and Shields29, Reference Papini, Maccheroni and Bruni38, Reference Sullivan, Watt and Barker41], confirmed cases [Reference Wilson, Philpott and Breer43] or confirmed cases and close contacts [Reference Andersen27]. One report did not detail who was treated [Reference Chan31]. Two reports also described a different treatment regimen for residents (ivermectin) and staff or other contacts (permethrin cream) [Reference Ladbury34, Reference Millership, Readman and Bracebridge35].
Excess death was reported by Barkwell et al. [Reference Barkwell and Shields29], in 6 months following ivermectin treatment. Four other reports of ivermectin use did not report this [Reference Dannaoui32, Reference Millership, Readman and Bracebridge35, Reference Paasch and Haustein37, Reference Sullivan, Watt and Barker41]. There were six reports of increased pruritus following scabies treatment with ivermectin [Reference Millership, Readman and Bracebridge35, Reference Sullivan, Watt and Barker41], Tenutex [Reference Moberg, Lowhagen and Hersle36], allethrin spray [Reference Paasch and Haustein37], lindane [Reference Parish39] and permethrin [Reference Yonkosky44].
Symptomatic relief
One report described the use of crotamiton cream for symptomatic relief of persistent pruritus following treatment [Reference Parish39], and another described re-treatment if pruritus persisted beyond initial anti-scabies treatment [Reference Paasch and Haustein37]. In two outbreaks crotamiton was used as part of the treatment regimen [Reference Barkwell and Shields29, Reference Chan31]; while other reports did not describe any symptomatic treatment for itch.
Environmental management measures
Ten reports described implementation of environmental measures to aid in outbreak control. Description of these varied widely, with some reports outlining protocols and others briefly stating RNC cleaning took place. The most commonly reported measures were ‘spray or disinfection of the infested persons’ environment’ and ‘washing of clothes’.
A summary of environmental management measures reported is presented in Table 4. Only two reports described protocols for the timing of these in relation to treatment [Reference Paasch and Haustein37, Reference Sullivan, Watt and Barker41]. Two others described repeat cleaning or infection control measures following treatment [Reference Andersen27, Reference Hetland33]. Despite reporting requiring additional drug treatment for treatment failures in seven of the 10 articles, only one reported repetition of environmental management measures in association with repeat treatment [Reference Dannaoui32].
Table 4. Summary of environmental management measures implemented in reports
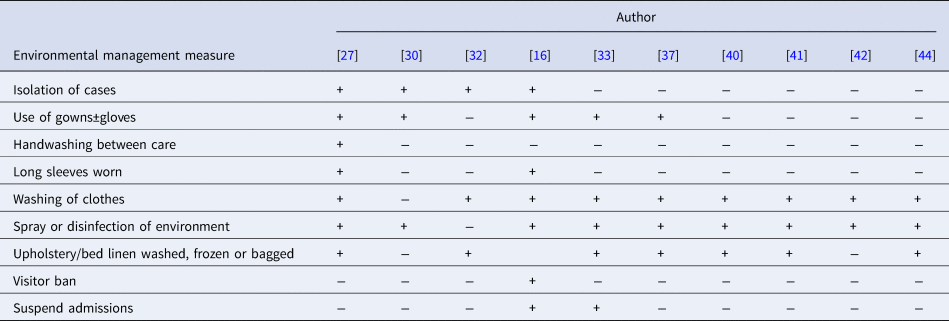
+ Represents measure implemented. − Represents measure not implemented or not described.
Where information regarding a measure was not provided in the text, the report was scored as not achieving said point (−).
Discussion
This systematic review reveals a moderate number of reports of the management of scabies outbreaks in RNC, but the absence of comparative studies assessing either drug treatment or environmental management. Permethrin was reported to be an effective treatment for scabies outbreaks in RNC, resulting in just five repeat cases. While lindane was also a commonly used topical treatment, further anti-scabies treatment was required in four of the seven outbreaks in which it was used. It was not possible to determine the effects of environmental management measures on any of the outcome measures in this review due to the lack of comparative studies. The quality of the data presented is low, and outcome measures used in previous literature (e.g. rate of treatment failure or duration of outbreak) were often missing. This limits the value of this observational evidence, which is much weaker than it need be. Outcomes within studies are poorly documented, with PROMs and PREMs almost entirely absent.
Consequently, no clear recommendations can be made about the effectiveness of drug or environmental interventions for outbreaks in RNC (beyond NICE guidelines for individuals). The recommendation in recent German guidelines [Reference Sunderkotter15], for permethrin with ivermectin as an alternative appear reasonable pending better evidence, especially given that individual comparison of lindane with permethrin shows it to be inferior [Reference Rezaee, Goldust and Alipour45]. There is clearly an urgent need for comparative, preferably randomised studies of the various drug treatments used, and these should include detailed description of all elements in the pathway from recognition of an index case through to evaluation of the effectiveness of outbreak control.
Despite the poor quality of data reporting, it was notable that delay in recognition or treatment of outbreaks was almost universally reported, with almost half of reports describing initial misdiagnosis. This is consistent with recent reports of RNC experience [Reference Hewitt, Nalabanda and Cassell6]. While RNC is a difficult setting to access for outbreak research [Reference Head46], which is necessarily done in a hurry, high-quality studies of interventions for infectious diseases in care homes can be achieved [Reference Cassell14, Reference Hayward47].
The poor data quality we observed also highlights the importance of using comprehensive and consistent data collection strategies to describe and analyse delay. This could be through the framework we present in Figure 1, which has been operationalised in our multi-outbreak report describing the clinical features of outbreaks in RNC [Reference Cassell14]. The quality assessment tool we adapted from the STROBE checklist used to capture the quality of observational data in this review may also be more widely applicable, to better differentiate studies where randomised controlled trials or controls are lacking.
It is less clear how an evidence base for environmental measures should be developed, especially given the uncertainty about drug treatments with which they will necessarily interact. Experiments on the survival of the scabies mite off its human host under the range of environmental conditions found in the RNC setting could inform the maximum time period after contact when decontamination is worth considering, while veterinary data may address in part the likely increased transmissibility of crusted scabies. A recent review by Arlian et al. [Reference Arlian and Morgan48] concluded that disinfection should not be necessary and isolation of the bed, bedding and clothing for 48 h should be sufficient. However, this would be challenging in RNC, and little evidence exists to support this from human studies. This is an urgent question for RNC – one report identified drug treatment as just one-fifth of the cost associated with the outbreak, with the majority of cost due to staff overtime, disposable gowns and gloves, cleaning supplies and laundry services [Reference De Beer16].
As so often in reports of infectious disease outbreaks, these case reports did not systematically address PREMs which are essential in the development of guidelines as they assess patients' objective experience of care and determine acceptability of a management strategy from a patient perspective. Although PREMs may be difficult to assess in this setting where many residents may be cognitively impaired, they must still be considered and reported where possible. In six reports increased pruritus was noted as a side effect following scabies treatment with a range of drugs. It is recognised that itch may persist for several weeks following scabies treatment [Reference Johnston and Sladden11], yet few reports recognised this and failed to describe whether symptomatic treatment was given alongside anti-scabies treatment. Drug treatments used for itch include topical crotamiton, topical hydrocortisone or oral antihistamines [19]. In order to address resident comfort and reduce suffering, symptomatic relief should be considered in future evidence-based guidelines.
In many outbreaks all residents, staff, family and contacts were treated and multiple treatment failures were ascribed to the lack of a highly-coordinated management plan. Whilst there are no UK national guidelines on scabies outbreaks in RNC [Reference White17], NICE guidelines for other cases of scabies infestation recommend simultaneously treating all household and close contacts [19]. Despite this advice, a recent systematic review by Fitzgerald et al. [Reference FitzGerald, Grainger and Reid49] found there is currently no evidence for the use of prophylactic treatment to prevent infestation in contacts. Patterns of exposure are likely to differ between family households and RNC, and the question of contact treatment should be addressed in future comparative trials of drug treatments.
One report by Barkwell et al. [Reference Barkwell and Shields29] described an increase in mortality in 6 months following ivermectin therapy in RNC residents. This cross-sectional study comparing deaths in a fixed period after ward level topical treatment and ivermectin mass treatment in two long stay wards has been heavily criticised due to the failure to control for factors leading to ward allocation and other confounders including other treatments, and it is not possible to conclude any increase in mortality is the result of ivermectin therapy [Reference Reintjes and Hoek50, Reference Coyne and Addiss51]. These results have not been replicated in any other study of ivermectin in RNC [Reference Dannaoui32, Reference Millership, Readman and Bracebridge35, Reference Paasch and Haustein37, Reference Sullivan, Watt and Barker41, Reference Coyne and Addiss51], a drug which is used worldwide in the mass treatment of river blindness (onchocerciasis) and serious adverse effects reported are rare [21].
This study reports only peer reviewed studies and it is possible that outbreak reports in the grey literature of similar quality may have been missed by our search strategy. We only included studies reported in English, and it is possible that comparative studies were missed. We did not review veterinary studies or experiments on mite survival which may be needed to specify and describe environmental interventions.
Overall, this review has highlighted a lack of comparative studies assessing the optimal drug treatment or necessary environmental management measures for outbreak control in RNC. Current observational evidence is weak and the evidential value of future studies could be improved through better reporting of outbreak outcome measures, such as time to diagnosis and time to effective treatment, as these incorporate any diagnostic delay or delay in effective treatment, which was common within reports. Patient-reported outcome and experience measures of treatment are important in the consideration of future national guidelines for managing these outbreaks and should be prioritised in future studies.
Conflict of interest
None.
Author contributions
Conception and study design (EJM, JAC, JM and SL), study search, article identification and data collection (EJM and JEC), quality assessment (EJM), development of conceptual framework (EJM and JAC) and drafting the manuscript (EJM). All authors reviewed and contributed to multiple drafts and agreed on the final version.