INTRODUCTION
Legionella infection is associated with two well-recognized distinct clinical and epidemiological forms: Legionnaires' disease (LD), which is a severe type of pneumonia, and the self-limiting non-pneumonic disease Pontiac fever. LD is a notifiable condition in most countries, and community-acquired LD occurs both sporadically and in outbreaks. LD accounts for up to 8–14% of cases of hospitalized community-acquired pneumonia [Reference Braun1, Reference Sopena2]. There is a high degree of geographical variation in the incidence of LD [Reference Bhopal3]; some of this variation may be due to differences in surveillance and diagnostic methods [Reference Bhopal3]. Known sources of infective aerosols include evaporative cooling towers, fountains, showers, nebulizers, and whirlpool spas [Reference Bhopal3]. In addition to these exposures, host factors are important for LD; these factors include age, gender, smoking, underlying illness and general immunodeficiencies [Reference Marston, Lipman and Breiman4].
While the epidemiology of LD is fairly well understood, there is limited knowledge concerning less severe and subclinical Legionella infections. Seroepidemiology is a suitable methodology to address this question. The aim of the present study is to analyse the overall Legionella antibody prevalence and changes in antibody titres during 1 year in a healthy general population. To examine risk factors for a positive Legionella serology or seroconversion, we analysed the association between Legionella antibody titres and self-reported health or possible environmental exposures. To our knowledge, changes in Legionella antibody levels during 1 year in a healthy population and the possible association with health outcomes and exposure variables have not been previously described.
MATERIALS AND METHODS
Blood samples were collected from 708 healthy, unpaid volunteer blood donors aged between 18 and 65 years living in the towns of Randers and Vejle in Jutland, Denmark. Generally, most blood donors give blood at least once a year. We have previously shown that there were no differences in Legionella seroprevalence between the two towns [Reference Rudbeck, Molbak and Uldum5]. For this study, the first sampling period was from late February to early June 2004 and the second one from February to June 2005. The median age of the blood donors was 45 years and 57% were males. There was no significant difference in age distribution (analysed in four age groups) or gender between donors in the two towns.
After 1 year, all returning blood donors (n=522) had a new blood sample taken and were asked to complete a questionnaire about socioeconomic variables, relevant exposures and health conditions during the previous year. A total of 27% of the blood donors had ceased donating blood because of age, pregnancy, health problems, moving to another area or unwillingness to continue to be a donor. The drop-out rates did not vary between the two towns.
The blood samples were analysed for Legionella antibodies by an indirect immunofluorescence antibody test (IFAT). Plate-grown, heat-inactivated Legionella (L.) pneumophila serogroups (sg) 1–6, L. micdadei and L. bozemanii were used as separate antigens. All sera were tested against all antigens. This assay is essentially the same as the well-characterized assay described by Wilkinson et al. [Reference Wilkinson, Fikes and Cruce6], which follows the guidelines of the United States Centers for Disease Control and Prevention (CDC). Specifically, antibodies to Legionella were detected with a fluorescein isothiocyanate (FITC)-conjugated rabbit anti-human IgM, A and G antibody (Dako F0200, Dako, Glostrup, Denmark). An E. coli-blocking fluid was used to block cross-reacting antibodies to other Gram-negative bacteria [Reference Bangsborg7]. The serum samples were titrated from 1:64 and upwards to end-point titre. A single titre of ⩾1:128 was used to define a positive Legionella antibody response; titres below 1:64 were not considered meaningful due to false reactions and background staining. The selected antigens are the antigens used for routine serological testing for LD at the Statens Serum Institut reference laboratory in Denmark and include the serogroups responsible for more than 80% of diagnosed LD cases (serogroups 1, 3, 6). Serogroups 2, 4 and 5 were included mostly for historical reasons, although serogroups 4 and 5 are not uncommon causes of LD (especially nosocomial LD). L. micdadei and L. bozemanii were included as they are the most common causes of non-pneumophila LD. The limitations, including cross-reactivity with other bacteria, of our method have previously been discussed [Reference Rudbeck, Molbak and Uldum5]. Cross-reactions between the Legionella antigens used are often seen; serological response to a Legionella infection is in general not serogroup specific. Infections with other serogroups than those used in the IFAT can on the other hand also be detected due to this cross-reaction. National laboratory test criteria for a confirmed diagnosis of Legionella infection include a ⩾fourfold rise in antibody titre to ⩾1:128 in IFAT (seroconversion) to L. pneumophila sg 1, 3, or 6. Seroconversion to other Legionella antigens and positive titres (⩾1:256) to any Legionella antigen are considered as presumptive of a recent or previous Legionella infection.
Risk factors examined included: type of residence; residence built before or after 1970; type of heating; presence of hot-water tank; hot-water tap-time (the tap-time was considered as slow if estimated not to be hot in ½-1 min); temporary stop in water supply; temporary uninhabited home; use of spa bath; showering elsewhere than at home; swimming pools, travel abroad; hotel stay in Denmark; summer cottage visits; and air-conditioning at work. Socioeconomic measures included previous education, job skills and total family income. Respondents were asked to provide information for the previous year including reporting the month of the possible exposure activity. Swimming pool use and showering outside the home were reported only if respondents engage in these activities regularly (several times a month).
A self-reported medical history for the past year was collected including questions about illnesses (influenza, pneumonia, common cold), hospitalizations, general practitioner (GP) consultations, absences from work due to illness and specific signs or symptoms (cough, fever, stomach pain, shiver, diarrhoea, headache, myalgia). The symptoms had to be present for at least 2–3 consecutive days in the previous year to be reported in the questionnaire.
The study was approved by the local scientific ethical committee (VF20030250) and the Danish Data Protection Agency.
Epi Data version 3 (Odense, Denmark) was used for data entry. Univariable analyses were conducted with antibody status as the dependent variable. Variables with a P value <0·2 were included in further multivariable logistic regression analyses about health and risk factors adjusted for age, gender, current smoking and place of residence (town). The described multivariable models were reduced to include variables with P values <0·1. The reference group was subjects with Legionella titres <1:128. The statistical analyses were done using Stata version 9.2 (Stata Corp, College Station, TX, USA).
RESULTS
In total, 522 subjects had blood samples available from 2004 and 2005 for testing and completed the questionnaire. One hundred and forty (26·8%) subjects had a Legionella titre ⩾1:128 in 2005. Seventy-one subjects (13·6%) had a titre ⩾1:128 to L. pneumophila sg 1. No subject had a titre >1:256. Thirty-six subjects had a ⩾fourfold rise in Legionella antibody titre to at least 1:128, corresponding to a 1-year risk of seroconversion of 6·9%. Conversely, 12 (2·3%) had a ⩾fourfold fall in titre from a titre of at least 1:128 after 1 year (Table 1).
Table 1. Fourfold changes in 1-year prevalence of Legionella antibodies in numbers in 522 healthy Danish blood donors tested twice within a 1-year interval, 2004–2005
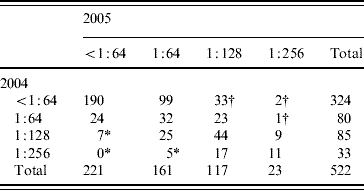
* Indicates fourfold fall in titre.
† Indicates fourfold rise in titre.
In Denmark, LD is a notifiable disease. All donors with a titre ⩾1:128 were searched for in the register for notified LD cases. None were found to have been reported with LD.
Self-reported health
Neither self-reported illnesses (influenza, pneumonia, common cold), hospitalizations, GP consultations, absences from work nor specific symptoms (cough, fever, common cold, malaise, stomach pain, shiver, diarrhoea, headache, myalgia) showed any significant difference between control subjects and cases where cases were defined either by a seroconversion to any Legionella antigen, a single antibody titre ⩾1:128, or a single antibody titre ⩾1:128 to L. pneumophila sg 1 (Table 2).
Table 2. Univariable analysis of self-reported health and risk factors in the previous year in persons with antibodies to Legionella pneumophila in Denmark, 2005Footnote *
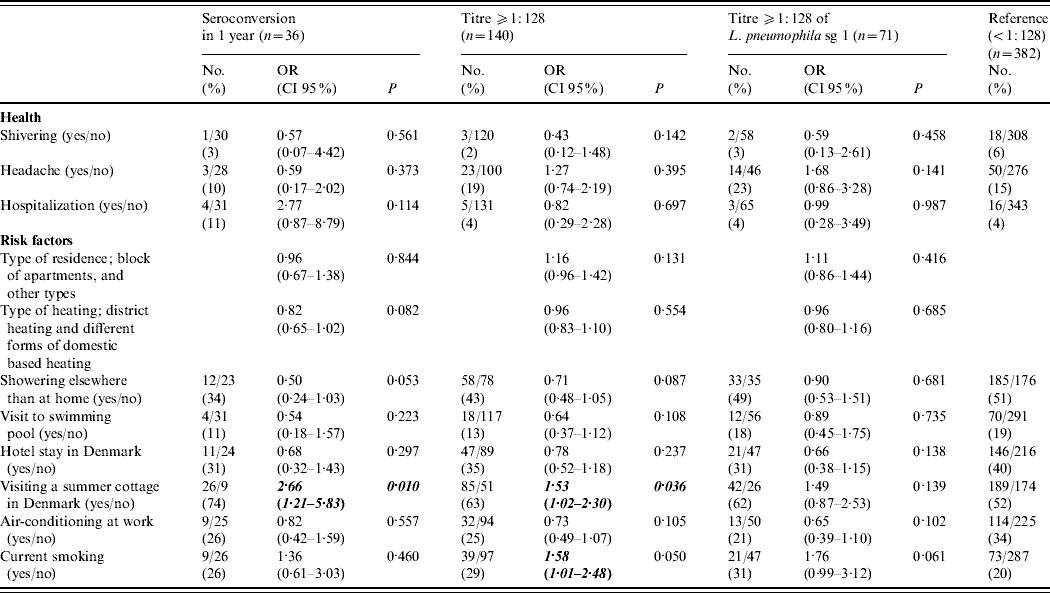
OR, Odds ratio; CI, confidence interval.
* The univariable analysis of the variables with P<0·2 in any of the three groups are reported.
There was no association between Legionella antibodies and a symptom complex of at least three, four or five symptoms of the eight common influenza-like symptoms (data not shown). In the multivariable models, no health-related variables were associated with being a case; this result was independent of the case definition (all P values >0·1).
Risk factors
Positive Legionella antibody titres or seroconversion were not associated with age, gender or town of residence (P>0·2 for all variables). Holidaying in Danish summer cottages was associated with an increased risk of Legionella seroconversion or a single positive Legionella antibody titre, but not for a positive Legionella sg 1 antibody titre ⩾1:128 (Table 2). In addition, current smoking was associated with increased risk of a positive Legionella antibody titre (Table 2).
The final multivariable model for Legionella seroconversion included visits to summer cottages [odds ratio (OR) 3·34, 95% confidence interval (CI) 1·48–7·55], showering outside the home (OR 0·41, 95% CI 0·19–0·88) and district heating compared with any form of domestic based heating (OR 0·75, 95% CI 0·59–0·96). The final multivariable model for a positive Legionella antibody titre included visits to a summer cottage (OR 1·61, 95% CI 1·07–2·43), and current smoking (OR 1·72, 95% CI 1·08–2·73). The final multivariable model for a positive L. pneumophila sg1 antibody titre only included visits to a summer cottage (OR 1·63, 95% CI 0·95–2·80) as a risk factor.
DISCUSSION
In 2005, the prevalence of a positive Legionella antibody titre in our study population was 27%. Thirteen percent of subjects were positive for antibodies to L. pneumophila sg 1, the most common cause of LD. During the course of 1 year, 6·9% of healthy, volunteer blood donors acquired Legionella antibodies; 2·3% of previously antibody-positive donors became negative for antibodies to Legionella.
In our population, visiting a Danish summer cottage was a significant risk factor for Legionella seropositivity and seroconversion. Current smoking also increased the risk for a positive Legionella antibody titre. We found no significant association between Legionella seroconversion or a positive antibody titre and travelling abroad, hotel stays or visits to swimming pools and spa baths. A positive Legionella serology or serconversion was not associated with an increased risk of self-reported absences from work, self-reported sicknesses or specific symptoms.
One potential limitation of our study is recall bias. We asked about common signs and symptoms that occurred during the previous year and these may not be easy to remember, leading to possible underreporting of symptoms. As study subjects were not aware of their Legionella antibody status, recall bias should affect both groups equally, probably resulting in a non-differential misclassification. Symptoms lasting only a short time which did not result in sick-leave absence or medical care may have been forgotten. We are reasonably certain of detecting only those episodes of illness that were of sufficient severity to be remembered. To increase the chance of detecting symptoms relevant for Legionella exposure, we analysed combinations of symptom complexes; these analyses did not affect the interpretation.
The lack of association between a positive Legionella serology and health complaints or self-reported sicknesses is consistent with the results in a Dutch outbreak where no health complaints were found in the seropositive group except for stomach ache, which may have been a spurious observation due to the large number of statistical tests done [Reference Boshuizen8].
Recall bias may have affected the collection of exposure variables as well, but probably to a lesser degree as most of the exposures are about everyday habits or activities that are easily remembered such as holidays and travel.
Our findings indicate that exposure to Legionella may be common. The possibility of repeated re-exposures in our population of continuously positive individuals cannot be ruled out, indicating that the yearly risk of exposure might be even higher than the number of seropositive individuals. Only a few subjects experienced a ⩾fourfold fall in Legionella antibody titres during the course of 1 year. The low number of subjects with a fourfold antibody fall in our population compared with the number with a fourfold antibody rise may be due to a change in exposure during the year or to the ‘trailing’ of antibodies, as losing antibodies takes longer than acquiring antibodies if infected. It might also be a chance finding.
In an outbreak in The Netherlands, both the percentage of high-normal antibody levels (>75th percentile) and high antibody levels (>99th percentile) was found to increase with exposure to L. pneumophila [Reference Boshuizen8]. Increased antibody levels were found in studies of exposed groups of hospital staff in nosocomial LD outbreaks as well [Reference Saravolatz9]. These findings suggest a dose-dependent effect on antibody response, indicating a dose-dependency in getting LD [Reference O'Brien and Bhopal10]. However, a study has failed to show a consistent relation between duration of exposure, frequency of exposure, or distance from the source and risk of LD [Reference Greig11]. This study suggested that transient exposure was sufficient to cause infection [Reference Greig11]. Nevertheless, most studies indicate that there are associations with either duration, frequency, or distance from source [Reference Brown12, Reference Den Boer13]. The present study corroborates the notion that exposure to and infection with Legionella is common. However, except for visiting summer cottages and current smoking, we were unable to pinpoint clear risk factors for Legionella serconversion or a positive Legionella antibody titre. Legionella are known to be widespread in the environment in Denmark [Reference Jeppesen14, Reference Brydov15]. Enclosed or stagnant water systems are associated with Legionella growth and the risk for LD [Reference Straus16]; these conditions are often present in summer cottages.
To our surprise, we did not find an association with travelling abroad, even though travelling abroad accounts for about 20–30% of the notified LD cases in Denmark [Reference Uldum and Krause17], and has been reported to be an independent risk factor in LD [Reference Den Boer, Nijhof and Friesema18].
No socioeconomic risk factors were associated with a positive Legionella antibody titre. However, our study population consisted of a voluntary, healthy population that was mostly middle class and therefore not particularly socially or economically diverse. The present study also was not designed to identify comorbid illnesses as risk factors as blood donors tend to be a healthy population.
In conclusion, there was a large and fairly constant number of individuals with Legionella antibodies in Denmark, and a considerable proportion of healthy Danish people develop Legionella antibodies during the course of 1 year without any measurable illness. These results indicate that environmental exposures to Legionella spp. in Denmark are common but seldom result in measurable morbidity in otherwise healthy adults.
ACKNOWLEDGEMENTS
We thank Helle Rieck and Marian Jørgensen for language revision.
DECLARATION OF INTEREST
None.




