INTRODUCTION
Studies in sub-Saharan Africa have shown that distance to a healthcare facility is an important determinant of mortality in children aged <5 years [Reference Becher1–Reference Kazembe, Kleinschmidt and Sharp6] and also a determinant of hospital attendance for fever [Reference Ewing7], diarrhoea [Reference Rahaman8], or hospital admission with an acute lower respiratory tract infection (ALRI) for children [Reference Weber9]. Only a few studies have taken account of seasonal differences while evaluating access to healthcare facilities [Reference Schoeps3, Reference Kazembe, Kleinschmidt and Sharp6, Reference Ewing7, Reference Blanford10] or the quality of care that they provide. None of the studies have evaluated the influence of distance to a healthcare facility on observed incidence of WHO-defined clinical pneumonia [Reference Gove11] or radiological pneumonia [Reference Cherian12], and vaccine efficacy (VE) against radiological pneumonia.
Radiological pneumonia is an important endpoint of trials of vaccines against respiratory infections [Reference Cutts13–Reference Klugman15]. The chance of detecting radiological changes in a child with pneumonia may be influenced by the stage of the illness at presentation, and hence by the ease of access to a healthcare facility. Children who live near a healthcare facility may, on average, seek care for pneumonia at a relatively early stage, before the development of consolidation, while those living further away are more likely to report when consolidation has developed. If a vaccine is efficacious, its effectiveness in reducing radiological pneumonia will be more apparent in this group. Understanding the relationship between geographical access to healthcare facilities and the clinical characteristics of invasive pneumococcal disease and pneumonia is important.
The differences in perceived risk and corresponding gain from visiting different tiers of healthcare services with different levels of function, staffing, and provision of clinical care may result in differential utilization of healthcare services and outcome for different diseases. The impact of distance to different tiers of healthcare facilities on observed mortality, incidence of clinical and radiological pneumonia, and VE against radiological pneumonia in children has not been studied in a comprehensive population-based prospective cohort study.
In most of sub-Saharan Africa it is difficult to conduct a cohort study because of absence of reliable information on births and deaths and also because of poor public health infrastructure. The pneumococcal vaccine trial conducted in a rural area of The Gambia from 2000 to 2004 [Reference Cutts13] provided a unique opportunity to conduct this cohort study because of the establishment of a system of registration of births and of surveillance systems for pneumonia and mortality. We investigated the following hypotheses in this cohort study.
-
• Mortality rates increase with increasing distance from the nearest healthcare facility.
-
• Observed incidence rates of clinical and radiological pneumonia fall with increasing distance from the nearest healthcare facility.
-
• Observed VE against radiological pneumonia increases with increasing distance from the nearest healthcare facility.
METHODS
Study area
The 9-valent pneumococcal conjugate vaccine trial (PVT) study area included almost all of the Upper River Division (URD) and Central River Division (CRD) of The Gambia, an area of about 500 km2 (Fig. 1). The climate is highly seasonal with a wet season between July and November and a dry season between December and June. A tarmac road runs almost parallel to the south bank of the river and connects the main healthcare facilities on the south bank. A network of dirt roads connects the rest of the area; some of these become impassable in the rainy season. Most people walk to the nearest clinic. The main modes of transport for people living further away are bush taxi, donkey carts and, occasionally, scooters or bicycles. Most families live in mud-walled and thatched-roof houses. Subsistence farming is the main socioeconomic activity.
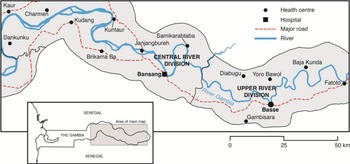
Fig. 1 [colour online]. Map showing fixed healthcare facilities in Upper and Central River Divisions of the The Gambia which participated in the surveillance of pneumonia in the Gambia Pneumococcal Vaccine Trial.
During the period of the trial, care for sick children was available free of charge from Government-run mother-and-child health (MCH) services, provided through 15 fixed facilities (at least once per week) and about 110 additional outreach clinics (up to four visits per month) (Fig. 1). The larger facilities, run by doctors, nurses and other auxiliary staff, provide outpatient and limited in-patient services, while the smaller ones have no resident doctor and provide only outpatient services. A village health worker (VHW) and traditional birth attendant provide primary care in rural settlements with a population of ⩾400 persons. VHWs have been trained to recognize and treat ALRI in children. Access to antibiotics from private practitioners or pharmacies is very limited. During the trial, PVT staff based at the two largest healthcare facilities in the area, Bansang Hospital (BN) in CRD and Basse Health Centre (BS) in URD, provided 24-h paediatric care with oxygen, broad spectrum antibiotics, radiographs, laboratory investigations including blood cultures and microscopy for malarial parasites, and blood transfusion. There was no fee for consultation at MCH clinics or BN/BS. There was no system of health insurance in place.
The PVT
The methods used in the PVT have been described previously and are summarized briefly below [Reference Cutts13]. This was an individually randomized, double-blind, placebo-controlled trial undertaken in URD and CRD. Infants who presented to a Government vaccination post and were resident in the trial area were eligible for enrolment in the trial. Infants who intended to move out within 4 months, had already received a dose of diphtheria-pertussis-tetanus-Haemophilus influenzae type b (DPT-Hib) or DPT vaccine, were aged <40 days or >364 days, had participated in a previous vaccine trial or had a serious chronic illness were not eligible. During the trial 17 437 children were randomized to receive three doses of 9-valent pneumococcal conjugate vaccine (PCV) given with DTP-Hib vaccine or DTP-Hib vaccine with placebo. Enrolment began in August 2000 with active surveillance of mortality and enhanced passive surveillance for pneumonia. All children enrolled in the trial were followed-up until they were aged 30 months or up to 30 April 2004, or until death or withdrawal from the trial, whichever was earlier. The median duration of follow up was 87 weeks.
Detection of cases of pneumonia and deaths
‘Radiological pneumonia’ (primary endpoint pneumonia), as defined by the WHO [Reference Cherian12], was the primary endpoint of the PVT; ‘clinical pneumonia’, defined according to the WHO criteria for ALRI [Reference Gove11], was a secondary endpoint and all-cause mortality was an additional endpoint.
Health-facility-based surveillance was conducted at BN and BS for clinical pneumonia and invasive pneumococcal disease (IPD). Outreach sites of BS were visited regularly by study personnel from the beginning of the PVT and, from April 2003, outreach sites of BN and seven additional healthcare centres were visited regularly. Children suspected of having pneumonia or IPD were referred to BN/BS for investigation by study nurses and doctors using standard operating procedures, including a chest radiograph for children with clinical pneumonia (radiographs were not available elsewhere). Deaths were monitored by 3-monthly home visits and at BN/BS. The sensitivity of mortality surveillance was therefore high and equal across the study area while the sensitivity of pneumonia surveillance varied according to the chance of the child attending BN or BS, which was expected to vary by distance.
Mapping
Hand-held global positioning systems (GeoExplorer III, Trimble Navigation, USA) were used to record the longitude and latitude of residential compounds in the catchment areas of BN/BS and areas between them on the south of the river. Straight-line (Euclidian) distances from a compound to both the nearest outreach clinic and to the nearer of BN/BS were calculated using ArcGIS 9·2 software (ESRI, USA). In addition, an isotropic time surface approach was used to incorporate effects of existing transport routes and barriers to movement on travel time [Reference Tanser16]. We assessed the use of theoretical travel time as an alternative indicator of access, but straight-line distances and modelled travel times in the study area were highly correlated (Pearson's correlation coefficient, r = 0·79). Since travel time can vary greatly by season, availability of transport, physical fitness of the carer, etc., we present results based on straight-line distance.
A number of studies have used 5 km as the cut-off for assessing the association between distance to a healthcare facility and the risk of mortality [Reference Van den Broeck, Eeckels and Massa2, Reference Armstrong4–Reference Kazembe, Kleinschmidt and Sharp6]. Therefore, we categorized straight-line distance as <2, 2–5 and >5 km in order to estimate risk for mortality and pneumonia.
Statistical analysis
The main endpoints for this analysis were rates of all-cause mortality, clinical and radiological pneumonia and VE against radiological pneumonia. A piecewise exponential survival model was used to estimate the effect of distance on time to first episode of each endpoint and to test whether observed VE (1 – rate ratio) against radiological pneumonia was modified by geographical accessibility to radiographs at BN/BS. We assumed that the hazard was approximately constant in each age-calendar time band. By choosing this model we did not have to choose a functional form for the hazard. We compared the results of the piecewise exponential model to those of the Cox proportional hazard model, and found that the results were very similar for both models. We present the results of the piecewise exponential model since it is conceptually simpler. We accounted for clustering in compounds by using robust estimates of standard errors. A Wald test was used to test for an interaction between child age and distance to healthcare facility (i.e. the proportional hazards assumption).
Children contributed to follow-up time from the date of first vaccination until the outcome of interest, end of PVT or date of withdrawal. Follow-up was partitioned by age, period of surveillance and season. In the regression models, we included PCV vaccination status, gender, and ethnicity as baseline characteristics, and periods of surveillance, season and age groups as time-varying covariates. A Kaplan–Meier analysis was used to estimate the cumulative incidence of mortality and pneumonia as a function of follow-up time. For simplicity we neglected death as a competing risk for pneumonia. However, since deaths are relatively rare, the estimate is a good approximation of pneumonia risk. We used Stata v. 12.0 for Windows (StataCorp LP, USA) for our analyses.
RESULTS
Characteristics of the study children
In the PVT, 8489 children were enrolled in the catchment areas of BN and BS and in the area between them; 6938 (82%) children had data on residential compound coordinates and were included in this analysis (Table 1). The children lived in 3136 compounds. The average distance between a residential compound and the nearer of BN/BS was 11·2 km (s.d. = 6·7, range 0·1–27·1).
Table 1. Baseline characteristics of all study children (N = 6938)
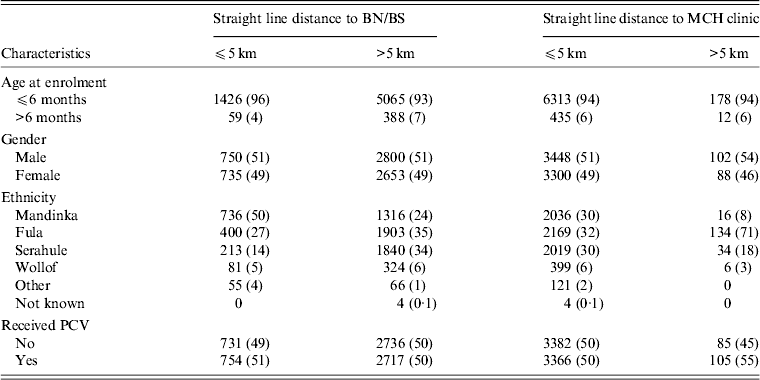
BN, Bansang Hospital; BS, Basse Health Centre; MCH, Mother and Child Health; PCV, pneumococcal conjugate vaccine.
Values given are n (%).
Most (79%) children lived >5 km from BN/BS, whereas only 3% lived >5 km from the nearest MCH clinic (including outreach clinics) (Table 1). Distance from BN/BS was greater for children aged >6 months at enrolment, and varied by ethnic group but not by gender. The distance to a MCH clinic did not vary by age or gender but a significantly higher proportion of Fula lived >5 km from a MCH clinic compared to other ethnic groups (P < 0·001).
Number of deaths and first episodes of clinical and radiological pneumonia
There were 370 deaths; 176 (47·6%) occurred in PCV-vaccinated children and 175 (47·3%) occurred in male children (Table 2). The median age at death was 14 months (interquartile range 9–20 months). Two hundred and twenty-nine (62%) deaths occurred in the rainy season. There were 3264 first episodes of clinical pneumonia and 542 of radiological pneumonia, 1934 (59·3%) and 241 (44·5%) of which were reported in the rainy season.
Table 2. Rates per 1000 child-years and 95% confidence intervals of all-cause mortality, clinical pneumonia and radiological pneumonia in all study children by risk groups
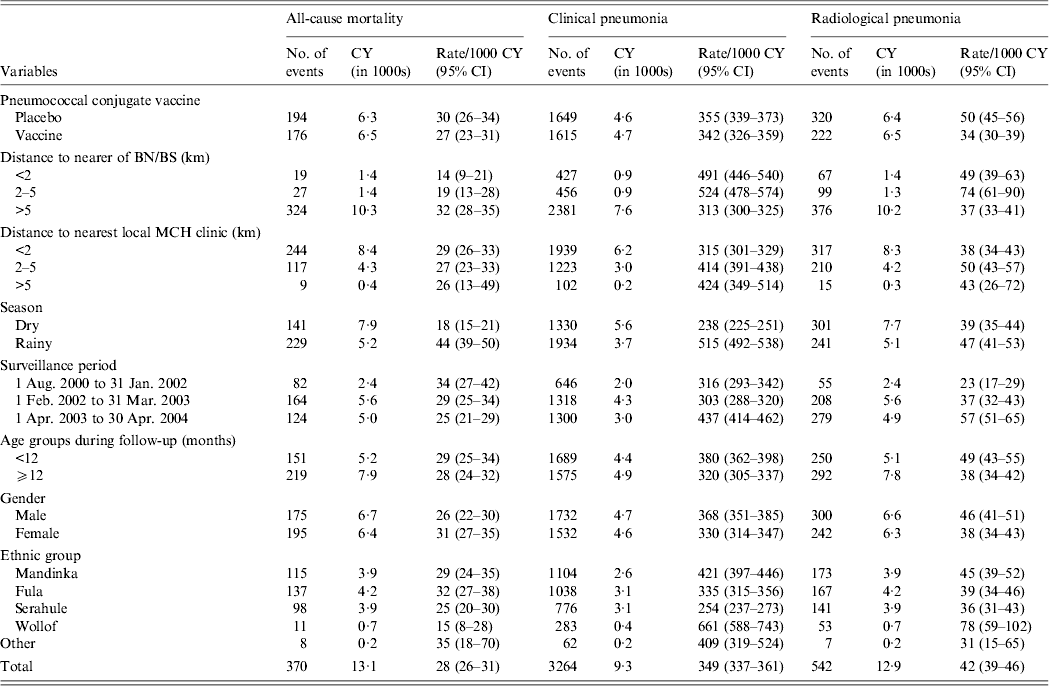
CY, Child-years; CI, confidence interval; BN, Bansang Hospital; BS, Basse Health Centre; MCH, Mother and Child Health.
Influence of distance to BN/BS or a MCH clinic on mortality
Distance to BN/BS
Mortality in control children increased with distance from BN/BS up to 11–15 km then fell to the rate observed at 6–10 km (Fig. 2 a). The estimated probabilities of death during ∼2 years of follow-up in control children living ⩽5 km vs. >5 km from BN/BS were 3·8 [95% confidence interval (CI) 2·7–5·5] and 6·5% (95% CI 5·5–7·5), respectively (Fig. 3 a). Similar, significant differences were found in PCV-vaccinated children (data not shown).
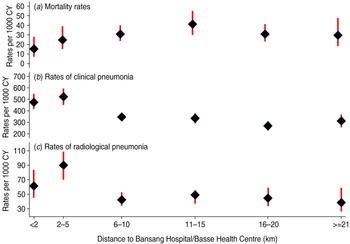
Fig. 2 [colour online]. Mortality rates, observed rates of clinical and radiological pneumonia with 95% confidence intervals in control (unvaccinated) children by distance fom their residential compounds to the nearer of Bansang Hospital or Basse Health Centre.
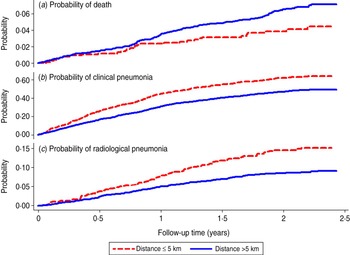
Fig. 3 [colour online]. Kaplan–Meier survival curve showing the probability of death, clinical pneumonia and radiological pneumonia in control (unvaccinated) children by follow-up time and distance from their residential compounds to the nearer of Bansang Hospital or Basse Health Centre.
Mortality in all study children (PCV-vaccinated and control), was significantly higher in children living >5 km from BN/BS than in those living nearer to BN/BS (Table 2). The mortality rate in children living >5 km from BN/BS was 2·78 (95% CI 1·74–4·43) times higher than in those living within 2 km (Table 3).
Table 3. Risk factors for all-cause mortality and observed pneumonia (number of children included in the analysis = 6938)
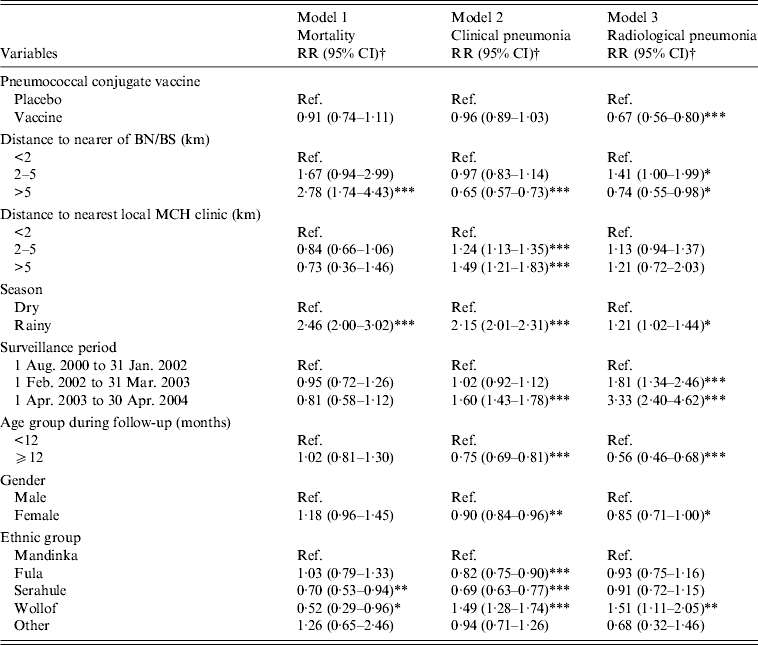
RR, Rate ratio; CI, confidence interval; Ref., reference group; BN, Bansang Hospital; BS, Basse Health Centre; MCH, Mother and Child Health.
† Adjusted rate ratios and 95% confidence intervals from a piecewise exponential survival model that included all variables simultaneously.
* P < 0·05, ** P ⩽ 0·01, *** P < 0·001.
Influence of distance to BN/BS or a MCH clinic on observed rates of clinical and radiological pneumonia
Distance to BN/BS
Rates of both clinical and radiological pneumonia in control children increased up to a distance of 2 km then decreased with increasing distance to BN/BS, but the rates flattened off after about 5 km (Fig. 2 b, c). The estimated probabilities of detection of clinical pneumonia in control children living ⩽5 km vs. >5 km of BN/BS during 2 years of follow-up were 62% (95% CI 58–65) and 46% (95% CI 44–49), respectively (Fig. 3 b). Corresponding figures for radiological pneumonia were 14·5% (95% CI 12·1–17·4) and 8·5% (95% CI 7·5–9·7), respectively (Fig. 3 c). Similar significant differences were found in PCV-vaccinated children (data not shown).
The observed rate of clinical pneumonia was 35% (95% CI 27–43) lower and that of radiological pneumonia 26% (95% CI 2–45) lower in all children living >5 km from BS/BN compared to children living within 2 km (Table 3).
Distance to MCH clinics
For all study children, the observed rate of clinical pneumonia increased with increase in distance to MCH clinics (Table 2). Compared to children living <2 km from the nearest MCH clinic, those living 2–5 km and >5 km from the nearest MCH clinic were 1·24 (95% CI 1·135–1·354) and 1·49 (95% CI 1·21–1·83) times, respectively, more likely to be diagnosed with clinical pneumonia (Table 3). Observed rates of radiological pneumonia were not statistically significantly associated with distance to the nearest MCH clinic (Tables 2 and 3).
Influence of distance to BN/BS on observed pneumococcal VE
Observed VE against radiological pneumonia was similar (33%, 95% CI 20–44) for children included in the current analysis to that reported previously in the entire study population (n = 17 437) [Reference Cutts13] and was not confounded by distance to BN/BS or to the nearest MCH clinic (Table 3). Observed VE was also not modified by distance to BN/BS or to the nearest MCH clinic (data not shown).
There was no evidence for an interaction between age and distance to the healthcare facility.
DISCUSSION
This study has shown that geographical access to healthcare facilities is an important determinant of care-seeking for pneumonia and of survival in children resident in a rural area of The Gambia.
Hypothesis 1, namely that mortality rates increase with increasing distance from the nearest healthcare facility, has been supported by the data. Mortality was higher in children living further from BN/BS than in those living nearer to these facilities, i.e. the only facilities where blood transfusion, oxygen and injectable broad spectrum antibiotics were available round the clock. Reporting bias is very unlikely since each death was ascertained actively in the community through 3-monthly home visits, with follow-up of reported deaths by field supervisors, and/or at the paediatric wards of BN/BS.
The observed positive association of distance to the nearest MCH clinic with observed incidence of clinical pneumonia could possibly be related to unmeasured confounding factors as we did not collect information on socio-demographic or environmental circumstances of children. This finding was contrary to our hypothesis that those living further away from a healthcare facility would less likely to seek care and therefore to be detected (one component of hypothesis 2). Laboratory support for diagnosis of malaria was not present in MCH and outreach clinics. Early treatment of malaria could have resulted in an apparent reduction in the incidence of clinical pneumonia in children living nearer to outreach clinics as, without the support of laboratory diagnosis, these conditions are often confused [Reference O'Dempsey17]. There was no association between distance to MCH clinics and observed incidence of radiological pneumonia. This was expected as there was no X-ray facility at any of the MCH clinics.
The effect of distance to BN/BS on lower observed incidence of clinical and radiological pneumonia was supported by the data. It is not possible to determine whether this lower observed incidence was due to lower incidence of pneumonia per se or because of lower ascertainment of pneumonia cases due to poor access to and utilization of services at BN/BS. The most likely reason for this lower observed incidence of pneumonia was reduced case ascertainment, as the further away children lived the less likely they were to see trial clinical staff, who could only visit peripheral outreach clinics a maximum of once or twice each month even during the last year of the trial. These findings are supported by the results of a previous study in The Gambia by Weber et al. who observed that incidence rates of hospital admissions of children with ALRI and severe respiratory syncytial virus infection were highest in areas closest to the study hospitals [Reference Weber9].
The data from this study did not support hypothesis 3, namely that distance from a healthcare facility influenced observed VE against radiological pneumonia. We had anticipated that children living close to a healthcare facility would receive treatment early in the course of their illness and be less likely to develop radiological pneumonia than those living further away, so that rates would be reduced in both controls and vaccinated children, reducing study power to detect VE. However, we found a lower observed incidence of radiological pneumonia in children living further away, which did not support our hypothesis. No association was found between increasing distance from healthcare facilities and observed VE against radiological pneumonia. The observed lower incidence of radiological pneumonia in children living further away from BN/BS suggests that a large proportion of children with advanced consolidation were not ascertained. This may have reduced our ability to detect an effect of distance on VE against radiological pneumonia.
The PVT did not record information on socioeconomic status of the parents of the trial participants. Therefore, we were unable to adjust the impact of distance on rates of pneumonia and mortality for differences in socioeconomic factors.
In our study we used straight-line distance as the measure of geographical access of a household to a healthcare facility. There are arguments that straight-line distance is a suboptimal measure for studies of this kind since it ignores physical barriers such as rivers, swamps, hills and traffic systems. However, in our study area, south of the river, patients did not have to cross any such barriers. In a study in Yemen, straight-line distance and driving time were strongly linked with vaccine uptake [Reference Al-Taiar18]. We have also found a strong correlation between distance from BN/BS and mortality or radiological pneumonia regardless of whether the distance was measured as straight-line or estimated travel time (data not shown).
The findings of this study show a strong and significant effect of distance to major healthcare facilities on mortality and the observed incidence of pneumonia, findings that are likely to apply to other areas, where people live in similar conditions and the availability of adequate healthcare services is poor. Conjugate pneumococcal vaccines may be especially valuable in such areas. In addition, improving access of sick children to interventions such as blood transfusion, appropriate antimicrobial and oxygen therapy by improving means of transport could help in reducing the burden of pneumonia and overall child mortality in many parts of rural Africa.
ACKNOWLEDGEMENTS
We thank all staff of Basse Field Station, Medical Research Council Unit, The Gambia for their assistance and all the study children and their families for their participation. This analysis was supported by the Johns Hopkins University with funds provided by the Boards of the Global Alliance for Vaccines and Immunizations and the Vaccine Fund. The PVT was funded by grants from the National Institute of Allergy and Infectious Diseases at the USA National Institutes of Health through contract N01-AI-25477; WHO through contract V23/181/127; the Children's Vaccine Program at PATH; and US Agency for International Development.
DECLARATION OF INTEREST
None.









