Introduction
Bordetella pertussis is the primary aetiologic agent of pertussis, a highly contagious, vaccine-preventable disease of the human respiratory tract [Reference Carbonetti1].
In Mexico, vaccination against pertussis started in 1954 with the use of an inactivated whole-cell vaccine (wP) that was used for more than 50 years in a five-dose scheme. Side effects of this vaccine led to the development of an acellular vaccine (aP) [Reference Sato, Kimura and Fukumi2] which was introduced in 2007 [Reference Perez-Perez3].
The current immunization schedule consists of four doses of a pentavalent acellular vaccine (DTaP/Hib/IPV) (Sanofi-Aventis de México, S.A. de C.V.) that contains pertussis toxin (PT) and filamentous hemagglutinin (FHA) toxoids as protective antigens and is administered at 2, 4, 6 and 18 months of age. The fifth and final dose, administered at the age of 4 years, is a booster with absorbed diphtheria, tetanus and whole-cell pertussis vaccine (DTwP) (Laboratorios de Biológicos y Reactivos de México, S.A. de C.V). This vaccine contains the inactivated B. pertussis strains Bp134, Bp509, Bp6224 and Bp25525 [4–7].
The increase in pertussis has been attributed mainly to improved diagnostic methods and the use of acellular vaccines which fails to protect against the colonisation and transmission of the aetiologic agent [Reference Mooi, Van Der Maas and De Melker8, Reference Warfel, Zimmerman and Merkel9]. On the other hand, it has been suggested that the whole-cell vaccine has led to the global emergence of ptxP3 positive strains, which have been associated with increased virulence of the pathogen [Reference Mooi10, Reference Bailon11] and more severe disease [Reference Bailon11, Reference Clarke12]. Also, it has been reported the appearance of isolates that present allelic variations among the main virulence factors used as vaccine antigens, such as pertussis toxin, FHA, pertactin (PRN) and fimbriaes (FIM) [Reference Bouchez13].
In the last decade, several countries reported an increase of pertussis cases, including Australia, the Netherlands, Spain, Japan, Canada, the USA, Peru and Brazil [Reference Bailon11, Reference Campbell14–Reference Rocha18].
In 2009 and 2012, the number of pertussis cases increased in various states of Mexico, amongst which: Sonora, Jalisco, Durango, Estado de Mexico and Nuevo León. In Nuevo León the pertussis incidence increased from 4.45 to 5.69 cases per 100 000 habitants [19].
In order to explain the reemergence of pertussis, we assessed the molecular epidemiology of B. pertussis isolates circulating in the population of northeastern Mexico.
Methods
Ethics statement
This study was performed in compliance with the requirements of the research and biosafety ethics committee of the School of Medicine of the ‘Universidad Autónoma de Nuevo León’ (Approval GA17-00001). Epidemiological and demographic data of the patients were included as part of a routine laboratory diagnosis. Therefore, no informed consent was required.
Study population and pertussis diagnosis
Symptomatic and asymptomatic cases were recruited from eight different public health jurisdictions of the secretary of health of Nuevo León, Mexico from the epidemiological week (EW) 52, 2006 to EW 52, 2014. The Centres for Disease Control clinical criteria for pertussis formed the guidelines to identify suspected cases of pertussis [20].
Of each suspicious pertussis case, a maximum of five personal contacts were recruited. Asymptomatic contacts were defined as those contacts with the detection of Bordetella spp. positive by culture and PCR without any symptoms.
Nasopharyngeal swabs were cultured on charcoal agar plates (BD, Franklin Lakes, New Jersey, USA) supplemented with 5% w/v defibrinated sheep blood and cephalexin (0.04 µg/ml) (Sigma-Aldrich, St. Louis, Missouri, the USA). Colonies with Bordetella morphology were phenotypically tested, i.e. Gram staining, catalase, oxidase, motility, urea hydrolysis, nitrate reduction, citrate utilisation and culture on MacConkey and blood agar. Species identification of the recovered isolates by culture and the samples of nasopharyngeal swabs were confirmed by real-time PCR [Reference Tatti21]. Recovered isolates were stored at −80 °C in brain-heart infusion broth containing 30% glycerol.
Demographic information was obtained from all patients with a positive pertussis diagnosis. Epidemiological data, such as case or contact, age and gender was recovered. Vaccination history was obtained from National Vaccination Card used in Mexico from each patient.
Pulsed-field gel electrophoresis (PFGE)
PFGE was performed on selected isolates. Isolates were grown for 72 h at 37 °C on charcoal agar plates supplemented with 5% w/v defibrinated sheep blood. Colonies were washed in 2 ml 0.85% w/v saline solution (3 min, 13 500 rpm) and the pellet was resuspended in 410 µl PIV buffer (1 M NaCl, 10 mM Tris-HCl; pH 7.4). After the addition of 150 µl 1.5% w/v molecular grade agarose, plugs were prepared from homogeneous mixtures.
The plugs were incubated at 37 °C for 3 h in 1 ml lysis solution (6 mM Tris (pH 8), 1 M NaCl, 100 mM EDTA (pH 8), 0.5% w/v, N-lauroylsarcosine, 0.2%, w/v, N-deoxycholate) containing 1 mg/ml lysozyme (Bio Basic, Inc. Ontario, Canada) and 50 µg/ml RNase A (US Biological). The lysis solution was replaced by 1 ml ES-buffer (100 mM EDTA (pH 8), 1% w/v N-lauroylsarcosine) with 0.1 mg/ml proteinase K (Bioline, USA Inc., Boston, MA. E.U.A.) and incubated overnight at 55 °C. After repeated washing (5 × 30 min. with agitation) of the plugs in 1 × TE buffer (10 mM Tris, 1 mM EDTA; pH 8), they were incubated for 12 h at 37 °C with restriction buffer containing 20 eU XbaI enzyme (Promega Fitchburg, Wisconsin, USA). Plugs were loaded on a 1.0% w/v agarose gel with 0.5 × Tris-borate-EDTA. The pulsed-field program was run on a CHEF-DRII system (Bio-Rad, Hercules, CA, USA) under previously described conditions [Reference Bisgard22]. Restriction patterns were visually compared with Lab-Works-IVP 4.5 software (Ultra-Violet products, Upland, Armonk, NY). The similarity coefficients of PFGE patterns were calculated using the Jaccard coefficient and SPSS v20.0 software.
Isolates with identical (i.e. 100% similar) restriction patterns were classified as a clone and strains that differed in 2–3 restriction fragments were classified as a subtype [Reference Tenover23].
Genotyping
To sequence the ptxP, ptxA, prn, fim2 and fim3 regions in selected B. pertussis isolates, DNA was extracted with the phenol-chloroform method and amplified by PCR using previously described oligonucleotides and conditions [Reference Schmidtke24, Reference Borisova25]. PCR products were purified by precipitation with 3 M sodium acetate and absolute ethanol at −20 °C. The products were sequenced at Macrogen, Korea. Sequencing reactions were performed in the DNA Engine Tetrad 2 Peltier Thermal Cycler (BIO-RAD) using the ABI BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The samples were injected to electrophoresis in an ABI 3730xl DNA Analyser (Applied Biosystems) and the sequences were compared on the Bio Edit platform (Biological Sequence Alignment Editor Platform).
Statistical analysis
Difference between vaccinated and unvaccinated cases was calculated using the Wilcoxon signed-rank test and the Z-test of proportions was applied to compare the clone A variations through the years with the SPSSv20 software. A P < 0.05 was considered significant.
Results
Study population and species detected
Among 11 864 nasopharyngeal swabs analysed, 687 (5.8%) were positive for pertussis; 244 (36%) were confirmed by both culture and PCR and 328 (48%) only by PCR (Table 1). The most frequently detected species were: B. pertussis (n = 665/687, 97%), Bordetella parapertussis (n = 16/687, 2.3%) and Bordetella bronchiseptica (n = 2/687). Four cases (n = 4/687) had a coinfection involving B. pertussis and B. parapertussis. We recovered a total of 359 clinical isolates (352 B. pertussis, 6 B. parapertussis and 1 B. bronchiseptica) during the study period.
Table 1. Study population included from 2006 to 2014, in the state of Nuevo León, Mexico
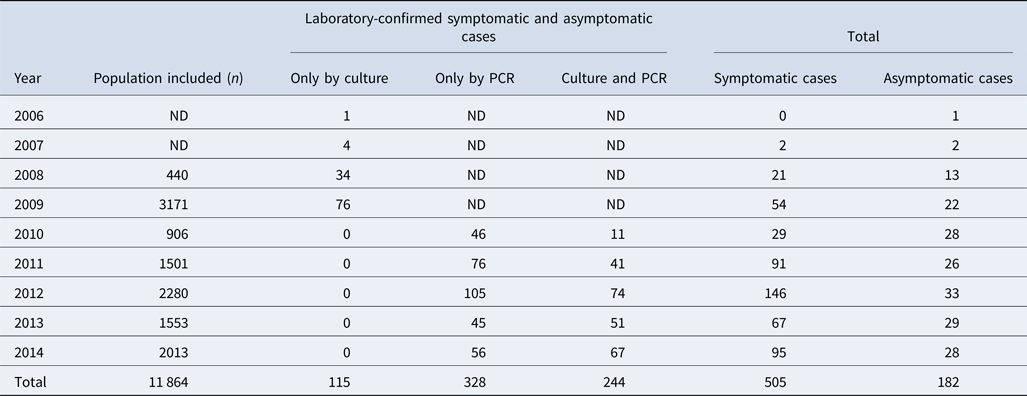
ND: no data because the PCR diagnosis was not performed. The PCR was implemented in 2010.
Epidemiological data
Epidemiological data were available from 359 cases: 265 symptomatic (74%) and 94 asymptomatic (26%). Among symptomatic cases, 142 (53%) were female and 123 (47%) were male. The mean age of symptomatic cases was 2 months (range 13 days–13 years). Interestingly, 55% (n = 94) of the children aged between 2 and 18 months had not received any aP vaccine at all, while 36% (n = 62) had received only the first dose (Table 2). In contrast, of the 94 asymptomatic cases, 68 (72%) were female and 26 (28%) were male. The mean age of the contacts was 24 years (range 10 months–77 years). More than 83% of asymptomatic cases should have had the complete immunisation scheme. However, only one participant had the complete scheme.
Table 2. Distribution of vaccination by age group of the symptomatic cases
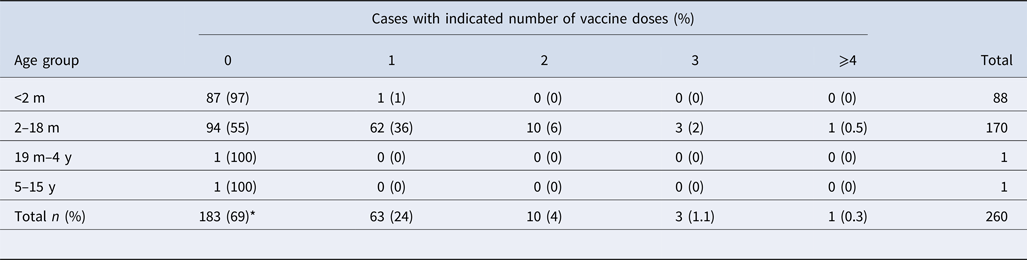
m, months; y, years; ND, no data available.
*P < 0.05 between vaccinated (n = 77) and unvaccinated (n = 183) patients.
Clonal diversity of the isolates
A total of 113 B. pertussis isolates recovered from both symptomatic (n = 81) and asymptomatic (n = 32) cases were selected for PFGE typing, using a simple sampling technique stratified by years (Confidence level CI 99%, Confidence Interval CI = 10). The total size of each stratum was calculated with the following formula: ni = n (Ni/N), where ‘n’ is the total size previously calculated (n = 113), ‘N’ is the total size of the population of the study (n = 352) and ‘Ni’ is the size of the stratum (number of the isolates collected by years). On this basis, we included 1 isolate from 2007, 11 from 2008, 23 from 2009, 4 from 2010, 13 from 2011, 24 from 2012, 16 from 2013 and 21 from 2014. DNA digestion generated a maximum of 15 restriction fragments and 12 pulsotypes with a similarity from 75 to 100% (Fig. 1).
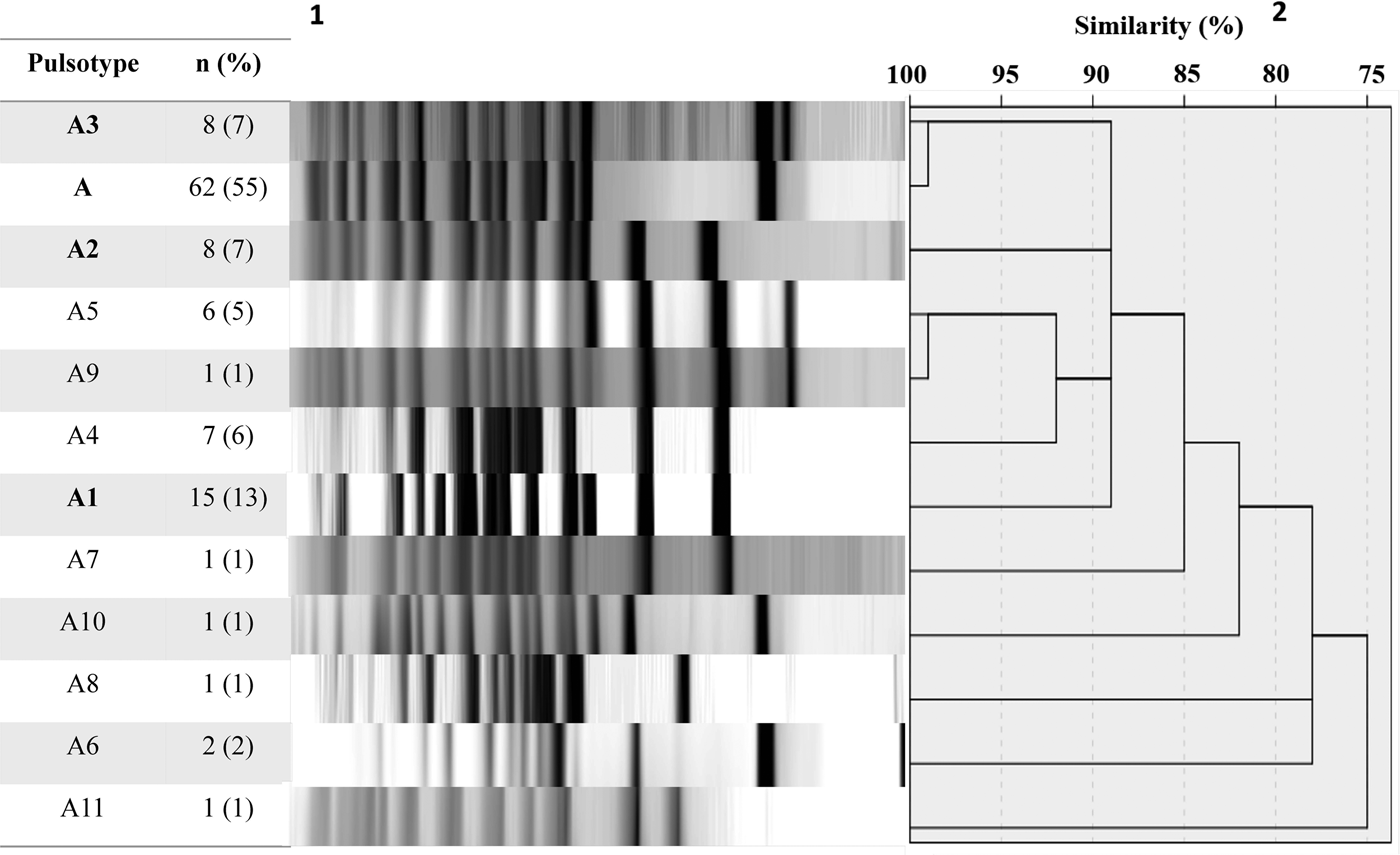
Fig. 1. Pulsed-field gel electrophoresis analysis of 113 B. pertussis isolates collected from 2007 to 2014. 1number and percent of isolates corresponding to each genotype. 2Similarity coefficient generated using SPSS 20.0 software.
Twelve different clones were identified; the predominant clone was designated A and three major subtypes were identified as A1, A2 and A3. Clone A represented 55% (n = 62/113) of the isolates and persisted from 2009 to 2014. The frequency of Clone A increased from 2% in 2010 to 37% in 2012 (P < 0.05) and to 21% in 2014. Subtype A1 persisted from 2008 to 2011 and increased from 33% in 2008 to 53% in 2009. Nevertheless, it was not detected in the following years (2012–2014). Subtype A2 was identified for 3 years (2008, 2009 and 2011) and was not identified in the following years. On another hand, Subtype A3 appeared from 2012 to 2014 while clone A decreasing. None of the predominant pulsotypes was detected in 2007 (Fig. 2).
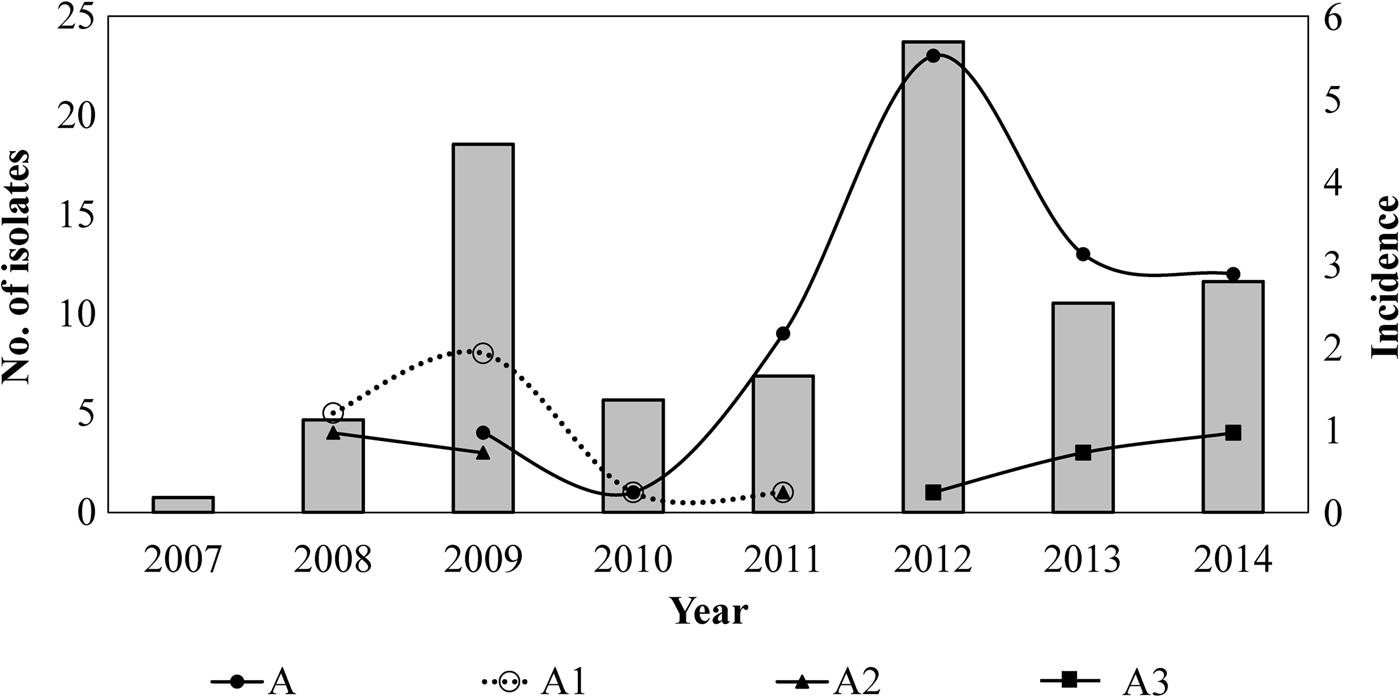
Fig. 2. Temporal relation of the hypervirulent clone (A) and subtypes (A1, A2 and A3) over the years. Bars represent the incidence of reported cases of pertussis (per 100 000 hab) by years, in Nuevo Leon, Mexico, from 2007 to 2014.
Among the selected isolates, just four of them were detected to be case/contact related with two belonging to clone A (recovered in 2008) and two to clone A1 (recovered in 2014).
Pulsotypes and vaccination status
Clone A and subtypes A1, A2 and A3 were isolated from both vaccinated and unvaccinated participants; 45% (n = 28/62) of all participants infected with clone A had not received any vaccine, 24% (n = 15/62) had received the first dose of aP vaccine and 1.6% (n = 1/62) had received three aP vaccine doses. Interestingly, none of the participants infected with clone A or the major subtypes had been given the fifth and final dose as recommended by the vaccination schedule.
Clone A was recovered from the eight health districts of the state of Nuevo Leon. It appeared in 2009 in the city of Monterrey (state capital) and spread from there to the other municipalities during the following years. On the other hand, subtype A3 was recovered from five health districts and subtypes A1 and A2 from three.
Typing of ptxP and ptxA regions
Sequencing yielded that 100% (n = 113/113) of isolates harboured the allelic combination ptxP3-ptxA1 (ptxS1A). A random selection of clone A and subtypes A1-A3 were typed for prn, fim2 and fim3 genes. The comparative analysis demonstrated the presence of ptxP3-ptxA1-prn2-fim2-1-fim3-2 (subtypes A1 = 2008 and A2 = 2009) and ptxP3-ptxA1-prn2-fim2-1-fim3-1 (Clone A = 2012, A3 = 2014) genotypes.
Discussion
This is the first report on the epidemiological and molecular analysis of B. pertussis isolates from a population of northeastern Mexico in a period from 2006 to 2014. We identified four representative pulsotypes (A, A1, A2 and A3) and displayed the dynamic and epidemiological changes of B. pertussis strains throughout the study period.
Subtypes A1 and A2 were displaced by clone A, which predominated during the 2012 outbreak. Clone A spread through the state of Nuevo Leon and became endemic for 6 consecutive years (2009–2014) in both the vaccinated (aP vaccine) and unvaccinated population. Interestingly, clone A was the only one that was distributed in all jurisdictions, which demonstrates its ability for statewide dissemination.
Previous studies have suggested that vaccination with the wP vaccine has led to the generation of strains presenting allelic and antigenic variants in the major virulence factors of the microorganism (prn, ptxP, ptxA, fim2, fim3, fha), as well as to the emergence of hypervirulent ptxP3 strains [Reference Bouchez13]. Our results are consistent with this idea. In Mexico, the wP vaccine was used for more than 50 years, which may have facilitated the appearance of ptxP3 strains in our population. However, this cannot be demonstrated because isolates were not collected before and during the period that wP vaccine was applied. Despite this, the ptxP3 lineage could be involved. Previous studies in other countries have shown the association between the appearance of ptxP3 strains and the resurgence of the disease [Reference Mooi10, Reference King26].
Interestingly, the endemic clone A showed the emerging genotype ptxP3-ptxA1-prn2-fim2-1-fim3-1 also known as MT27a [Reference Miyaji16]. In this genotype, the allele for the fimbria type changed from fim3-2 to fim3-1. This change has been associated with both the transition from wP to aP vaccination in countries where fimbrial proteins are included as antigens of aP vaccine [Reference Kurniawan27].
The appearance of emerging genotypes has agreed with the increased pertussis incidence in other countries as well seems to be related in Mexico [Reference Bailon11]. It has been shown that a large proportion of the isolates is harbouring the allelic combination ptxP3-prn2, lack expression of the PRN [Reference Lam28]. It is necessary to extend the studies to find out if PRN deficient isolates are circulating in Mexico.
Although this situation has been associated with the wide use of aP vaccine which includes PRN, these isolates were detected before the implementation of aP vaccination. Despite this, its appearance coincides with an increased incidence previously occurred in the USA and Australia [Reference Pawloski29, Reference Safarchi30]. Contrarily, in Mexico, FIM and PRN proteins are not included in the aP vaccination.
We believe that aP vaccination has contributed to the dissemination of the microorganism because only 2 years after the replacement of wP by aP there was a shift in the epidemiological pattern of the disease with outbreaks in 2009 and 2012 (years with the highest incidence rates in the state).
Besides, we detected a high level of incomplete vaccination schemes; only one participant had a complete vaccination scheme. This is an alarming situation because children and adults are the main sources of transmission to unvaccinated infants [Reference Conde-Glez31–Reference Wendelboe34]. The findings highlight the importance of rigorous evaluation of the vaccination scheme used in Mexico as well as the assessment of the effectiveness of the applied aP vaccine.
The observed increment in pertussis may also reflect the use of more sensitive and specific diagnostic methods. In Nuevo León, in the past, pertussis was confirmed by culture and phenotypic tests. In 2010, the use of dual-target real-time PCR was implemented [Reference Tatti35] and in 2011, a multiple target real-time PCR was used [Reference Tatti21]. The multiple target PCR allows the identification of B. parapertussis, Bordetella holmesii and a presumptive identification of B. bronchiseptica. Our data show that the implementation of multiplex PCR coincides with an increase in identified pertussis cases. Although this is a known phenomenon, it is worth mentioning because the diagnosis of pertussis by both phenotypic and genotypic methods is performed only in four Mexican states: Nuevo León, Sonora, San Luis Potosí and Chiapas [36].
This study has some limitations, e.g. the incomplete vaccination history data, the limited clinical data from patients; and that some symptomatic cases and related asymptomatic cases may represent the same strain and thus may introduce selection bias in the sampling.
In conclusion, the presence of an endemic clone and three predominant subtypes belonging to the genotypes ptxP3-ptxA1-prn2-fim2-1-fim3-2 and ptxP3-ptxA1-prn2-fim2-1-fim3-1 were detected. This finding supports the global spread/expansion reported for these outbreaks associated genotypes.
Acknowledgements
We thank Denisse Rodríguez Gómez for her kind assistance at the laboratory.
Conflict of interest
None.







