INTRODUCTION
Liver fluke disease, or fasciolosis, of livestock and humans is caused by endoparasitic trematodes of the genus Fasciola. Fasciola hepatica is responsible for the disease in temperate climates whereas F. gigantica is found in tropical zones. Fasciolosis may cause serious ill-health in humans, but rarely death [Reference Mas-Coma, Barques, Esteban and Dalton1]. Extensive haemorrhaging and inflammation occur as ingested fluke migrate to the bile duct through the peritoneal cavity and across the liver parenchyma. Feeding of mature fluke on the bile duct lining eventually causes hyperplasia and inflammation of the epithelium leading to thickening and dilation of the bile ducts and gallbladder. Every year an estimated 2·4 million people are infected worldwide and a further 180 million people are at risk of infection [Reference Mas-Coma, Barques, Esteban and Dalton1] with human infections reported in many countries including Iran, Peru, Cuba, Bolivia and Egypt [Reference Chen and Mott2–Reference Esteban10].
The highest prevalence of human fasciolosis is found in the Altiplano region of northern Bolivia where the incidence of disease in certain localities can reach above 50% [Reference Esteban10–Reference Strauss18]. However, none of the reports of fasciolosis in Bolivia are of sufficient scope or size to gauge the effect of fasciolosis in the whole region, to determine the areas of greatest infection or to assess the effect of contributory factors. Generating such data would require an extensive epidemiological survey randomly sampling the whole region for human and animal fasciolosis. This would be a major undertaking, and until such a survey can be performed, an alternative approach should be taken.
In this paper we have collated data obtained by us and other laboratories during surveys in the Bolivian Altiplano [Reference Esteban11–Reference Buchon20] to provide a dataset of considerable scope and size, and subjected this to a detailed analysis. This has provided a clearer picture of the incidence of fasciolosis in the region, and through statistical analysis techniques, such as chi-squared automated interaction detection (CHAID) [Reference Hill, Delaney and Roncal21, Reference Rakowski and Clark22], has allowed us to identify factors that influence disease spread, and the communities at highest risk. The analysis provides useful information that can be used to design future control programmes.
METHODS
Statistical analysis was undertaken using SPSS for Windows version 11 and the related package AnswerTree (SPSS Inc., Chicago, IL, USA). The data from ten surveys of the Altiplano in Bolivia were compiled and the data from 7908 individuals from 38 communities compiled into a single file with infection data associated with province, community size, community, age, 10-year age grouping and gender. χ2 automated interaction detection was used to assess the effects of province, community size, community and age grouping on infection rate. Logistic regression analysis was used to determine the effect of age on infection rate.
RESULTS
The Bolivian Altiplano is a high plain region situated between two Andean mountain ranges, ∼3700 m above sea level. The Altiplano is the largest expanse of arable land in the Andes and is inhabited principally by the indigenous Aymaran population. The incidence of human fasciolosis hepatica from 7908 individuals from 38 communities within these provinces surveyed over the last 11 years, and of bovine fasciolosis is shown in Figure 1. The overall recorded infection level (18·53%) and long study period (infection has been recorded from 1984) is indicative of an endemic infection. Human and bovine fasciolosis is associated with the communities lying in the plain from Lake Titicaca to La Paz, predominantly in the Los Andes province. Human infection levels >10% are restricted to the corridor of Batallas and the plain close to Lake Titicaca. In contrast, low infection rates of both animals and humans were found in areas on higher ground such as Chasquipampa in the Murillo province.
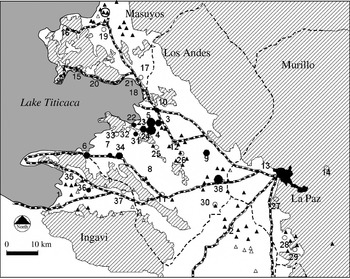
Fig. 1. Cattle and human infection in the Bolivian Altiplano. Communities: (1) Guaqui; (2) Viacha; (3) Calasaya; (4) Cutusuma; (5) Chijipata Alta; (6) Huacullani; (7) Quiripujo; (8) Caleria; (9) Coropata; (10) Batallas; (11) Tambillo; (12) Pucarani; (13) El Alta; (14) Chasquipampa; (15) Tuaca; (16) Santiago De Huata; (17) Coromata Baja; (18) Copancara, (19) Achacachi; (20) Huatajata; (21) Cuyahuani; (22) Kharapata; (23) Pantini; (24) Oketiti; (25) Iquiaca; (26) Ancocagua; (27) Achocalla; (28) Tuni; (29) Kajchiri; (30) Ticuyo; (31) Aygachi; (32) Belen Yayes; (33) Cohana; (34) Lacaya Baja; (35) Yanarico; (36) Chambi Grande; (37) Causaya; (38) Kallutaca; Data were obtained for a total of 7908 individuals from 38 communities in the Northern Bolivian Altiplano from four provinces (Los Andes, Ingavi, Omasuyos, and Murillo) around the La Paz region covering the period 1984–1998 [Reference Bjorland7, Reference Hillyer8, Reference Esteban11–Reference Buchon20]. Within these provinces, 25 communities from Los Andes, four communities from Ingavi, five from Murillo, and six communities from Omasuyos were sampled. Infection rates are represented by symbols: Open circles <10% human infection; small closed circles 10–25%; medium closed circles >25–40%; large closed circles >40%. The presence or absence of infection in cattle was obtained from 4750 cattle in 86 differing communities. Cattle infection is represented by triangles (open triangles, no infection; closed triangles, infection). Areas with gradients >3° are diagonally shaded. The scale bar represents 10 km.
Infection data from humans and cattle [Reference Buchon20] from the same 12 communities (899 cattle) showed a significant correlation (P=0·02) between the percentage of humans infected and the percentage of cattle infected (% human infection rate=0·769×% cattle infection rate+4·678). This observation indicates that human fasciolosis is a zoonosis associated with rural communities.
CHAID analysis on the effect of community size, province and community on infection level (Fig. 2) shows that human infection is significantly higher in communities with less than 2000 individuals (26·26%) than in larger communities (5·55%), confirming the designation of the disease as one of small rural communities. Infection in these 30 small rural communities was higher (34·06%) in the Los Andes province (where infection was recorded in 17 out of 18 communities examined) than in the Ingavi, Murillo and Omasuyos provinces (collectively 3·76% with infection recorded in only 5 out of 12 communities). Within each province infection levels differed significantly between communities with some such as Chijipata Alta and Cutusuma showing very high levels of infection (56·94%).

Fig. 2. The effect of province, community size and community on infection. CHAID (chi-squared automated interaction detection) was used to separate the population from the Northern Bolivian Altiplano into subgroups based on the proportion of infected and non-infected individuals. The predictor variables were province, community size (<2000, ⩾2000) and community. Numbering of the communities is consistent with that for Figure 1.
One factor that could contribute to the differences in infection levels between communities is the age profile of the population. Accordingly, for the 923 individuals for which the appropriate age data was available, CHAID was used to examine the age profile of infection within differing communities (Fig. 3). In all communities examined there was a significant effect of age grouping on infection level. Infection level decreased with age.
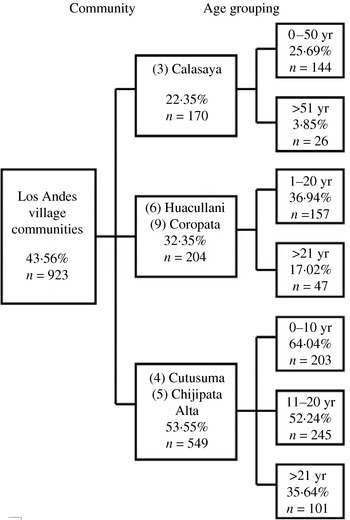
Fig. 3. The effect of community and age on infection. Data was available for the age structure of the samples (10-year age bands) for a subset of the village communities from the Los Andes province with CHAID used to assess the effect of community and age grouping on infection rate. Numbering of the communities is consistent with that for Figure 1.
Construction of a detailed age profile (Fig. 4) from 268 individuals from Calasaya, Cutusuma, and Chijipata Alta communities showed that peak infection was associated with children aged 8–11 years and progressively decreased thereafter with a very highly significant (P=0·0001) decrease in infection with age. No effect of gender on infection level was observed.

Fig. 4. Age profile of community infection rate in the communities of Calasaya, Cutusuma and Chijipata Alta. For analysis of the affect of age on infection rates, a subset of the data from three communities of Los Andes province with high infection rates was used. These were Calasaya (89 individuals), Cutusuma (150 individuals), and Chijipata Alta (29 individuals) (268 individuals in total).
DISCUSSION
Fasciola hepatica requires an invertebrate intermediate host, the mud snail Lymnaea truncatula, and a definitive mammalian host [Reference Andrews and Dalton23]. Fluke eggs released by infected mammals hatch, forming motile phototropic miracidia that invade the snail host to undergo several developmental and multiplicative stages that subsequently lead to the eruption of free-swimming cercariae. These cercariae settle and encyst on vegetation or remain on the water surface to form metacercaria that are infectious to mammals when ingested. In the duodenum of the mammalian host the parasites excyst as juvenile flukes migrating to the bile ducts where, within 4–5 weeks, they mature sexually. Here they can reside for up to 11 years producing eggs that are carried by bile into the intestine for defecation onto pastures [Reference Pantelouris24, Reference Dan25].
The prevalence of animal and human fasciolosis corresponds to snail distribution, which is restricted to the northwest of the Altiplano [Reference Mas-Coma, Funatsu and Bargues26, Reference Oveido, Bargues and Mas-Coma27]. Snail infection is promoted by animal reservoirs [Reference Hillyer28–Reference Ueno30] such as sheep, cattle, pigs, llamas and alpacas. Unlike its European counterpart the snail resides almost wholly sub-aqua and is observed on aquatic plants during the dry season [Reference Mas-Coma, Funatsu and Bargues26, Reference Oveido, Bargues and Mas-Coma27]. As a result, in the Altiplano, completion of the liver fluke life-cycle can occur even during the dry season, a time when animals and humans collect around the shrinking water sources. During the height of the rainy season, Lake Titicaca and its tributaries overflow causing extensive flooding, optimizing conditions for transmission. A positive relationship can be observed between the proximity to the lake and the prevalence of the fasciolosis in cattle and humans [Reference Hillyer8, Reference Mas-Coma, Funatsu and Bargues26].
In Europe larval development within the snail is halted in winter as low temperature influences the development of the free living and intra-molluscan stages of the life-cycle. Outbreaks recur in early spring as daytime temperature increases to >9°C. In contrast, temperature is not an important limiting constraint in disease transmission in the Bolivian Altiplano as all-year average night-time and daytime temperatures range from 0–6°C and 18–22°C respectively [Reference Mas-Coma, Funatsu and Bargues26, Reference Oveido, Bargues and Mas-Coma27].
The significant effect of cattle infection rate and community size on human infection rates reinforces the designation of fasciolosis in the Bolivian Altiplano as a rural problem. The conditions necessary to support endemic fasciolosis are predominantly associated with poorer rural communities where the majority of individuals residing in the Bolivian Altiplano are subsistence farmers relying on the land and their livestock to survive. Animals are primarily fed on aquatic plants and algae, which may be contaminated with F. hepatica metacercarial cysts. In some regions of the Altiplano pasturing is free or mixed and the absence of pasturing zones leads to contaminated pastures. Failure to treat infected animals due to a lack of affordable flukicide treatment further contributes to disease transmission.
Transmission in humans is believed to occur because of their dietary habits. Individuals supplement their diet with aquatic plants during daily animal husbandry [Reference Marcos31]. The main types of aquatic plants are berro berro (watercress), algas (algae), kjosco and totora [Reference Bjorland7]. Whilst drinking water from pumped sources is not believed to be associated with infection, the consumption of surface water while working in the fields is believed to be a source of infection. Accordingly, vegetables washed in contaminated water may also become a source of infection. The incidence of infection is almost inevitably aggregated within familial groups that share contaminated food and drink from a common water source [Reference Bjorland7].
CONCLUSION
It is apparent that human fasciolosis has been endemic to the Altiplano since at least 1984. This study highlights the need for a large regional control programme. This would be feasible since the area of highest infection is restricted to a corridor leading from La Paz to Lake Titicaca. Fasciolosis here is a true zoonoosis and mass treatment of animals would have a significant impact on human infection. A control programme would, therefore, need to involve a combination of large-scale drug treatment of F. hepatica-infected animals and humans, health education programmes and the introduction of better farm-management practices (or even separation of animals from water sources used by humans). Triclabenzdazole has been successfully used to treat both animal and human infection [Reference Laird and Boray32, Reference Apt33].
Health education programmes need to be directed particularly at children since these have a higher risk of F. hepatica infection [Reference Curtale34]. The higher level of infection in children may be related to an increased exposure to the infective stages of the parasite as children commonly work in the fields minding livestock where the intensity of transmission would be expected to be higher. It is also believed that children are more likely to eat aquatic plants [Reference Bjorland7]. Alternatively, the higher incidence of infection in children may indicate the existence of an age-related immunological resistance to infection by F. hepatica, a phenomenon that is well documented for the related digenetic trematode of the species Schistosoma [Reference Hagan35].
ACKNOWLEDGEMENTS
We acknowledge grants from Dublin City University, the Irish American Partnership, the Irish Health Research Board and Commission of the European Community Programme Life Sciences and Technologies for the developing countries (contract no. TS3-CT94-0294) in support of this research.
DECLARATION OF INTEREST
None.






