INTRODUCTION
Many zoonoses, including those caused by rodent-borne hantaviruses, are considered recent emerging or re-emerging infectious diseases [Reference Vorou, Papavassiliou and Tsiodras1]. In Europe, infection with Puumala virus (PUUV) in humans is responsible for haemorrhagic fever with renal syndrome (HFRS).
The primary reservoir is the bank vole [Myodes (Clethrionomys) glareolus] [Reference Bernshtein2]. Transmission of the virus to humans occurs mainly through the inhalation of infectious aerosols generated from saliva, urine, and/or faeces of these rodents [Reference Lähdevirta3, Reference Vapalahti4]. Handling wood piles and cleaning derelict buildings significantly increase the risk of hantavirus infection [Reference Crowcroft5]. In France, HFRS is endemic along the Belgian border, where six outbreaks have been reported since the 1990s: in 1993, 1996, 1999, 2001, 2003, and 2005 [Reference Mailles6]. However, the epidemic has recently spread south to the Franche-Comté region [Reference Heyman7], where genetic evidence of the circulation of PUUV among bank voles was recently described [Reference Artois8].
The presence and transmission of hantavirus depend on the distribution and infection of its animal hosts, and these factors largely determine the incidence and extent of HFRS [Reference Vapalahti4, Reference Schmaljohn and Hjelle9]. The bank vole is one of the most abundant mammal species in Europe, dwelling in and at the edge of forests [Reference Mazurkiewicz10]. It eats seeds, fruits of trees and bushes, and green plants. In the temperate broadleaf forest biome, the multi-annual bank-vole population dynamics are directly regulated by the seed production of trees, especially oak and beech [Reference Vapalahti4]. A high mast production in autumn means a higher food supply for bank voles, which in turn means a higher survival rate and earlier breeding throughout winter (particularly if the winter is mild), increasing rodent population densities in the early summer [Reference Tersago11, Reference Clement12]. However, in the southern part of the Franche-Comté region (montane forest biome), the M. glareolus population dynamics are more complex, exhibiting multi-annual synchrony with sympatric species such as Apodemus sp., Microtus arvalis, and Arvicola terrestris, the last two being grassland species. In these areas, predation and/or parasitism are the most likely driving forces behind the community population dynamics [Reference Giraudoux13, Reference Michelat and Giraudoux14]. In France, PUUV seroprevalence and abundance of rodents appear weakly linked [Reference Augot15]. A recent model suggests importance of the abundance of newly infected voles, due to the higher rate of infectious virus shedding in their excretions [Reference Sauvage, Langlais and Pontier16]. Climate conditions during the time of high bank-vole abundance can also increase the transmission of PUUV to humans because humid and colder conditions lead to better virus survival in the environment [Reference Kallio17].
The risk for developing HFRS also depends on human proximity, behaviour, and land-use patterns. Humans may be exposed to PUUV during the spring and summer when they participate in outdoor activities, or during cold winters if bank voles find shelter in houses. As a consequence, seasonal HFRS peaks could be better explained by shifts in rodent and/or human behaviour rather than rodent abundance or PUUV prevalence fluctuations.
Investigations assessing risk for HFRS must evaluate factors influencing the reservoir host population, the human population at risk, and potential factors driving their interaction. Therefore, the aims of this study were to characterize the space–time distribution of HFRS and to evaluate the relationship between HFRS incidence and environmental factors that potentially influence the PUUV reservoir abundance in the Franche-Comté region of France – the new epidemic area.
MATERIALS AND METHODS
Study area
The Franche-Comté region (16 202·34 km2, 1 117 059 inhabitants in 1999) is located in eastern France along the Swiss border. It is divided into 116 electoral wards or cantons whose populations range from 251 to 117 733 inhabitants, and sizes range from 6·54 to 339·46 km2. The main terrestrial biomes found in this region are temperate broadleaf forests in a large northern part and montane forests in a smaller southern part.
Incidence data
The surveillance of hantavirus infections in humans is conducted by the National Reference Centre for Viral Haemorrhagic Fevers (NRCVHF). Because the diagnosis of PUUV infection is complex, all serum samples that tested positive or borderline positive from any French laboratory are sent to the NRCVHF for mandatory confirmatory tests. A HFRS case is considered confirmed when one of the following conditions is fulfilled: detection of IgM and IgG antibodies (using IFA and ELISA methods); detection of IgM (without IgG) antibodies in an original sample and a marked increase in IgM levels in a follow-up sample; or detection of IgM (without IgG) antibodies in an original sample and evidence of IgG seroconversion in a follow-up sample.
The NRCVHF provided exhaustive data for HFRS cases diagnosed between 1999 and 2008 in people residing in the Franche-Comté region. Data were then aggregated at the canton level.
Demographic and socioeconomic data
Demographic data by canton, gender, and 5-year age groups were obtained from the 1999 census conducted by the French Office of Population Censuses. The expected numbers of cases for each canton were computed by applying an internal standard (i.e. incidence rates from the entire region for the same years) to the person-years of each area and stratifying by gender and 5-year age categories.
Landscape elements
Land cover determines the spatial distribution of animal reservoirs. A higher disease risk is expected in landscape mosaics of human-dominated and natural land covers, where contact between humans and rodent hosts is more frequent. The proportions of canton area covered by broadleaf forests (category 311), coniferous forests (category 312), mixed forest (category 313), and grasslands (categories 231, 321, 322) were extracted from the Corine Land Cover 2000 (CLC2000) database [18], using GRASS 6.4 [19]. Produced by the European Environment Agency, CLC2000 is based on the photo-interpretation of satellite images, and provides consistent information on land cover across Europe with a spatial resolution of 100 m.
Using the near-infrared and the red reflectance bands of a Landsat 7 ETM+ image acquired in September 1999, a mean normalized difference vegetation index (NDVI) was calculated for each canton to quantify its concentration of green leaf vegetation.
The average elevation of each canton was calculated using the digital elevation model from the GLOBE project [20].
Climate data
Average monthly meteorological data obtained from 97 temperature stations and 136 precipitation stations between 1999 and 2008 were provided by the French Meteorological Service (Météo France). Climate data at the centre of each canton (defined as the location of the main town) were estimated by residual kriging [Reference Joly21, Reference Joly22]. This approach was used to overcome the problems of non-stationarity, which prevents the direct use of ordinary kriging on observed meteorological elements. We assumed that non-stationarity and anisotropy could be explained by external factors. Therefore, we introduced independent predictors (elevation, slope, theoretical solar radiation, etc.) in forward stepwise regression models (monthly temperature and precipitation being the dependent variables) to describe the regional field. In a second step, the residuals were interpolated using ordinary kriging. Regression and kriging results were then summed to obtain the final estimates at the centre of each canton. These spatial data interpolations were carried out by using a specifically designed program written in C++.
Statistical modelling
Units considered in the statistical analyses consisted of the 116 cantons that compose the Franche-Comté region. To test for and locate the clusters of cases, we used the spatial scan statistic developed by Kulldorff et al. [Reference Kulldorff23]. We assumed that the numbers of cases were Poisson distributed. The method imposed a circular window on the map and allowed its centre to move over the area so that, at any given position, the window included different sets of neighbouring cantons. The window radius was varied continuously from zero to a maximum so that the window never included more than 30% of the total population. For each location and size of the scanning window, the null hypothesis was that the risk of HFRS was the same in all windows (corresponding to complete spatial randomness), whereas the alternative hypothesis was that there was an elevated rate inside compared to outside the window. For hypothesis testing, we used a Monte Carlo procedure to generate 99 999 random replications of the dataset under the null hypothesis. We also conducted a space–time scan test that was based on a cylindrical window with a circular geographic base and whose height corresponded to time. Time intervals were 1 month long, and the maximum temporal cluster size was 30% of the study period as a whole. We used this upper limit to optimize the test for clusters of small to medium size, in accordance with some a-priori epidemiological knowledge (rare disease driven by local environmental factors). Calculations were performed with the SaTScan software program, designed specifically to implement the spatial scan statistic [Reference Kulldorff24].
We studied associations between environmental risk factors and incidence rates of HFRS by Bayesian hierarchical models. Logarithms of observed and expected cases were linked with a set of covariate values in a linear model that accounted for Poisson errors. All independent variables (land cover, NDVI, elevation, climate) were fitted as continuous variables. To model the covariate effect in the presence of extra-Poisson variation, a spatially unstructured random effect (also called heterogeneity) and a spatial random effect (also called clustering) were introduced. For the spatial component, a conditional autoregressive (CAR) structure was used, imposing a dependence structure on the random effects [Reference Besag, York and Mollie25]. Two cantons were considered correlated if and only if they shared a common boundary.
Two parallel chains were run. A burn-in of 10 000 iterations was allowed, followed by 100 000 iterations for which parameter values were stored. Bayesian summary statistics, posterior density plots, chain history, and plots of Gelman & Rubin tests provided no reason to suspect lack of convergence.
The deviance information criterion (DIC) was used to compare models (models with smaller DIC are better supported by the data). Odds ratios (ORs) and their 95% credibility intervals (CIs) were calculated. Bayesian models were run with WinBUGS software [Reference Lunn26].
RESULTS
A total of 113 HFRS cases (84 males, 29 females) were identified in the Franche-Comté region between 1999 and 2008, corresponding to a mean incidence rate of 1·0/100 000. These notified cases originated from 54 of the 116 cantons. The mean age at diagnosis was 40·2 years (standard deviation=14·1 years).
HFRS infections displayed inter-annual fluctuations. A distinctive epidemic occurred in 2005 (40 cases) and was characterized by a summer peak (16 cases in June, 12 cases in July) (Fig. 1). This peak was preceded by a hot August month 2 years earlier (mean temperature 23·5°C), a cold July month 3 years earlier (17·8°C), and a moist November month 3 years earlier (mean precipitation 309 mm).
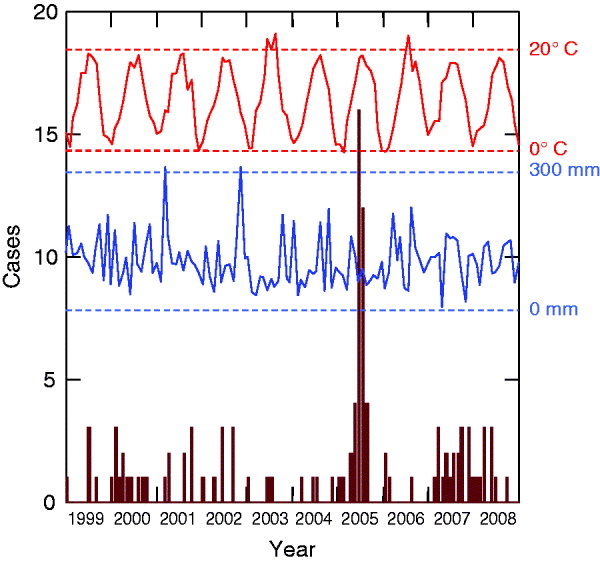
Fig. 1. Monthly cases of haemorrhagic fever with renal syndrome cases (in brown) and climatic profiles (temperatures in red, precipitation in blue) between 1999 and 2008 (Franche-Comté, France).
In a purely spatial analysis, one significant cluster was detected (Fig. 2). It consisted of 14 cantons (116 394 inhabitants) located in the south of the region (Jura, a sparsely populated and densely forested area). In this area, 50 cases occurred when 11·72 cases were expected (P=10−5), corresponding to an annual incidence rate of 4·3/100 000.
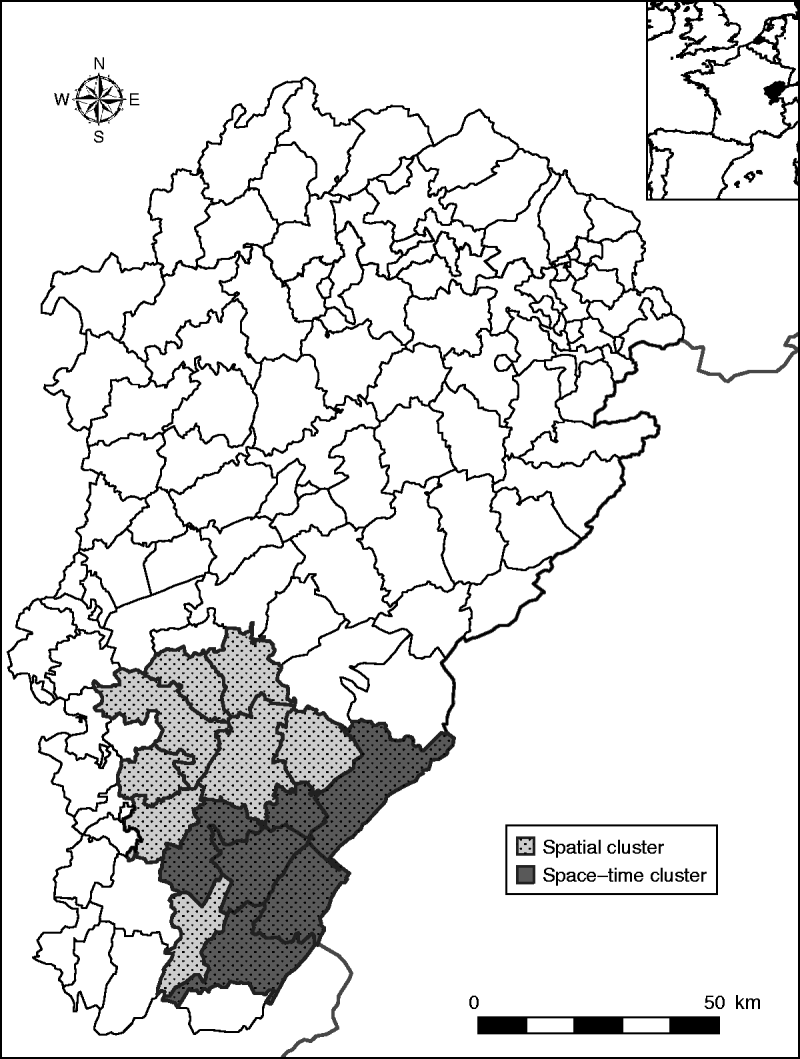
Fig. 2. Clusters of haemorrhagic fever with renal syndrome cases in the Franche-Comté region, France (light grey area: spatial cluster, 14 cantons; dark grey area: space–time cluster, six cantons, May–August 2005).
With the space–time approach, one significant space–time cluster embedded in the spatial cluster was also detected (Fig. 2). It corresponded to six cantons (52 227 inhabitants), where 29 cases occurred in May–August 2005 when only 0·19 cases were expected (P=10−5), corresponding to an annual incidence rate of 166·6/100 000.
When we conducted the univariate Bayesian regression analyses, only one significant explanatory variable was found: NDVI was positively associated with HFRS incidence (P=0·03, DIC=277·26) (Table 1). In a multivariate approach, the best model included the NDVI and the coniferous forests (P=0·07, and P=0·24, respectively), but the goodness-of-fit was not improved (DIC=277·18).
Table 1. Environmental risk factors for haemorrhagic fever with renal syndrome (univariate analyses, Franche-Comté, France, 1999–2008)
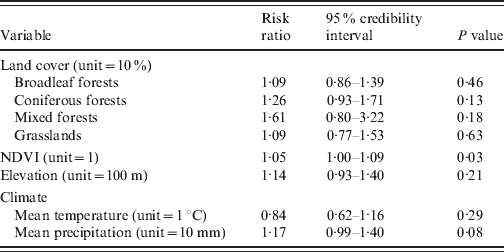
NDVI, Normalized difference vegetation index.
DISCUSSION
The analysis of HFRS in the Franche-Comté region between 1999 and 2008 clearly indicated space and space–time clustering. Moreover, our landscape epidemiology approach showed an association between a variable reflecting biomass (NDVI) and incidence of PUUV infection in humans.
Some limitations must be considered in interpreting our results. Diagnosing hantavirus diseases depends on clinical awareness because of the pauci-symptomatic or asymptomatic clinical course, and the relatively non-specific symptoms seen in a number of patients. As a result, some cases may have escaped the surveillance system, but we assumed that such under-reporting (if any) would have been space–time independent. HFRS data, which were based on the national referral system, provided no information about the location of contact with rodents or their excreta. However, given the mean size of the cantons and the known risk behaviours, it is likely that for most cases, the place of residence and the place of exposure were located in the same canton. Only a case-control study will allow a more detailed analysis of site-specific risk factors and include additional information about presumed locations of PUUV transmission occurrence.
Tree seed production by beech and native oak species was not available at the relevant spatial scale, and to date, no objective data on bank-vole population dynamics exists in France. However, we assumed that climate parameters represented adequate proxies for temperature-dependent mast formation and vole winter survival [Reference Clement12]. Known as important driving forces, climatic variations were not accounted for in the Bayesian regression models. These models would have required climate time-dependent data (which were available) and the number of observed cases per canton per year. Unfortunately, the limited numbers of HFRS cases per year precluded this approach.
Our results indicated no cyclic pattern in HFRS incidence in the Franche-Comté region and revealed a single-year (2005) epidemic state rather than an endemic state. This observation was in contrast to the patterns observed in the French Ardennes region [Reference Mailles6], Germany [Reference Koch27, Reference Hofmann28], and Belgium [Reference Tersago11, Reference Clement12]. Most of the HFRS cases occurred in early summer (June and July 2005), possibly when increased outdoor summer activities brought people into closer contact with bank voles or their excreta.
The influence of meteorological conditions on the 2005 peak is in line with results reported by Tersago et al. [Reference Tersago11] and Clement et al. [Reference Clement12]. They found that outbreaks can be predicted by high autumn temperatures, high summer temperatures, and a rather cold and moist summer, 1 year, 2 years, and 3 years, respectively, prior to the outbreak. High temperatures in year-2 might trigger bud formation on broadleaf trees, which results in heavy masting in year-1, resulting in HFRS peaks in year 0. In 2003, a heat-wave struck West-Central Europe. In Belgium, the heat induced the largest mast production (autumn 2004) ever recorded, and subsequently resulted in the highest HFRS peak (2005) observed thus far. As the Franche-Comté region has the same biotope in its large northern part (deciduous broadleaf forests), the same documented presence of M. glareolus, and the same recent climate changes, the local HFRS outbreak in 2005 might similarly be the result of the heat-wave that occurred in summer 2003 and the moist autumn in 2002.
However, a hot summer seems to be necessary, but not sufficient, to drive the risk of PUUV infection in humans, as the hot summer in 2006 (mean temperature 23·1°C in July 2006) was not followed by an incidence peak 2 years later. This finding may be explained by a mechanism proposed by Linard et al. to help understand the complex pathways between the environment and disease risk [Reference Linard29]. A high abundance of rodent hosts (driven mainly by landscape configuration, mast production, and community processes, i.e. host ecology) is not sufficient for transmission to occur. Beyond a host abundance threshold, environmental factors that influence the infection prevalence in the host population and favour the indirect transmission path (driven mainly by winter temperatures and soil moisture, i.e. virus ecology) could better predict the number of HFRS cases in humans. If the critical threshold is not met, risk areas are likely be localized, and cases would be sporadic. Moreover, some environmental factors specific to the terrestrial montane biome could be driving forces behind the space–time cluster detected in 2005 in the Jura mountains.
Within the most affected area (the space–time cluster), we observed an annual incidence of 166·6/100 000, which is higher than the maximum incidence reported in southern Germany (90/100 000 in 2007) [Reference Piechotowski30] and highlights the magnitude of the local epidemic in 2005.
The association between NDVI and HFRS incidence, also found by Yan et al. in China [Reference Yan31], supports the importance of green biomass. The biomass could potentially impact the transmission of hantavirus in two different ways: influencing the abundance of bank voles (tree seeding and suitable habitat) and/or influencing the contact within bank-vole populations (virus transmission). However, our study produced, one surprising observation: coniferous forest was the most significant land cover variable (P=0·13), but it is not a preferred habitat for bank voles in Western Europe [Reference Piechotowski30]. In our study, most cases were reported from mid-altitude areas where coniferous forests are largely dominant. This result may reflect human risk behaviours related to an increase in forest-related activities in cantons with a large forest cover because, to our knowledge, no studies have shown or even suspected differential behaviour of the bank vole in coniferous and deciduous forest habitats. On the other hand, broadleaf forests (considered the favorite habitat of bank voles) were not associated with the HFRS risk, in contrast with observations reported by Linard et al. and Schwarz et al. [Reference Linard32, Reference Schwarz33].
To minimize threats to public health and to maximize proper actions, attempts to forecast HFRS outbreaks are of the utmost importance. Unfortunately, the reasons that explain why the disease recently emerged in the Franche-Comté region remain largely unknown, and climate parameters alone do not reliably predict an HFRS epidemic in this area. To better understand bank-vole population dynamics as well as resulting epidemiological patterns and risk factors of human hantavirus infections, concerted efforts that combine reservoir monitoring, surveillance, and investigation of human cases are warranted.
DECLARATION OF INTEREST
None.





