INTRODUCTION
Kaposi's sarcoma (KS) is a tumour associated with significant morbidity and varied presentations [Reference Bagni and Whitby1]. Traditionally, KS has been categorized based on its clinical manifestations – classic, AIDS-related, African endemic, and iatrogenic subtypes. In classic KS, cutaneous lesions have little to no visceral involvement. In contrast, African endemic KS progresses rapidly with systemic involvement. AIDS-related KS represents the most aggressive form of the disease, and it has both cutaneous and visceral involvement. The fourth subtype is primarily iatrogenic and occurs secondary to severe immunosuppressive drug regimens following organ transplantation [Reference Gazouli2, Reference Rosenberg and Biggar3].
Herpesvirus 8 (HHV8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV), was identified as the causative agent in KS in 1994 [Reference Biggar, Curtis, Cote, Rabkin and Melbye4]. The mode of transmission has not yet been fully elucidated [Reference Osmond5]. Cross-sectional epidemiological studies confirmed that HHV8 seropositivity strongly correlated with a population's risk of developing KS [Reference Mesri, Cesarman and Boshoff6]. HHV8 has been found to be associated with both AIDS-related and classic forms of KS [Reference Melbye7].
Before the AIDS epidemic, KS was primarily endemic in the Mediterranean countries, especially Italy and Greece, and in Africa [Reference Beral8, Reference Chang9]. Classic KS was originally described in men of Mediterranean, eastern European, or Ashkenazi descent, which suggests a possible genetic predisposition to the tumour [Reference Jaffe, Bregman and Selik10]. Peak incidence occurred in the sixth decade of life, and patients usually experience a chronic course, with a prognosis of 10–15 years of additional life expectancy [Reference Eltom11]. At present, treatments for endemic KS have not been well studied and vary from observation to localized radiotherapy, surgical excision, and/or systemic chemotherapy [Reference Biggar12].
Although KS was rare in the USA prior to 1980s, the onset of the AIDS epidemic in the early 1980s brought a surge in the incidence of AIDS-related KS [Reference Biggar13, Reference Jacobson14]. The immunosuppression resultant from AIDS increased the risk of KS by nearly a thousand-fold [Reference Laor and Schwartz15]. Although several papers have examined the incidence of KS in European nations, few comprehensive studies that analyse both AIDS-related and classic KS epidemiology and survival in the USA exist [Reference Mocroft16–Reference Engels18]. In this study, we analysed the incidence, survival, and geographical distribution of AIDS-related and classic KS in the general US population, as represented in the Surveillance, Epidemiology, and End Results (SEER) database, between 1975 and 2005.
METHODS
Data source
The data for classic and AIDS-related KS incidence between 1975 and 2005 were obtained from nine population-based cancer registries that participated in the SEER tumour registries since 1973. The SEER-9 registry used included Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, California; Seattle-Puget Sound, Washington; and Utah. Included in the SEER areas were populations living in areas with a high incidence of AIDS, such as San Francisco, Seattle, Atlanta, and Hawaii. Areas with intermediate incidence of AIDS included Detroit, Connecticut, and New Mexico. Iowa and Utah had lower incidence of AIDS. This study employed a publicly available data source and was deemed exempt from further review by the UC Davis Institutional Review Board.
Classification of subtypes
In accordance with classification methods from a previous study [Reference Williams19], we classified KS cases as belonging to the ‘classic subtype’ if the affected individuals were diagnosed with KS during 1975–1979 (pre-AIDS era) or if patients were aged >70 years at time of diagnosis from 1980 to 2005. Patients aged <70 years at time of diagnosis from 1980 to 2005 were considered to have ‘AIDS-related KS’. Support for this means of classification includes the following previous studies. Classic KS accounted for 91% and 100% of the cases of KS in men and women aged >55 years, respectively, in one national study [Reference Dal Maso17], and for 93·9% of the cases of KS in persons aged >60 years in another study [Reference Shiels20, Reference Shiels21]. Moreover, because some experts found that as opposed to earlier occurring KS, the increase in KS seen in individuals aged 70–79 years included ‘almost no AIDS cases’ [Reference Shiels20], our method of utilizing a threshold of age 70 years is a reasonable threshold for delineating classic and AIDS-related KS in populations.
Statistical considerations
Age-adjusted standardized incidence ratios (SIR) were calculated by using the 2000 US standard population and expressed as the number of KS cases/100 000 people per year. The SEER*Stat software program, version 6.5.1 (Surveillance Research Program, National Cancer Institute, Bethesda, MD, USA) was used for data collection and analysis. Confidence intervals (CI) for the 5-year relative survival rates were analysed using Poisson distribution.
RESULTS
Demographics
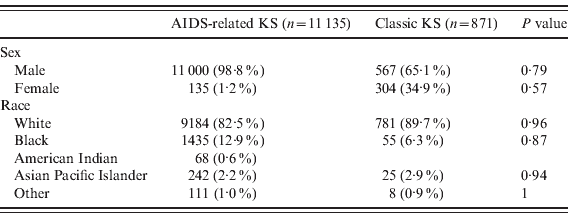
Between 1975 and 2005, 12 006 cases of KS were diagnosed in nine geographical areas of the SEER tumour registries. Of these, 871 cases were classified as classic KS, and the remaining 11 135 were identified as AIDS-related KS (Table 1).
Table 1. Demographics for AIDS-related and classic Kaposi's sarcoma (KS)
Trends in incidence of AIDS-related and classic KS in the USA
The overall SIR of AIDS-related KS (1·9) from 1980 to 2005 was not significantly higher than that of classic KS (1·4) from 1975 to 2005 (P = 0·78). While the overall SIR for each form of the disease during this time period was similar, a more meticulous examination of the annual SIR data reveals two distinct trends (Fig. 1a, b).
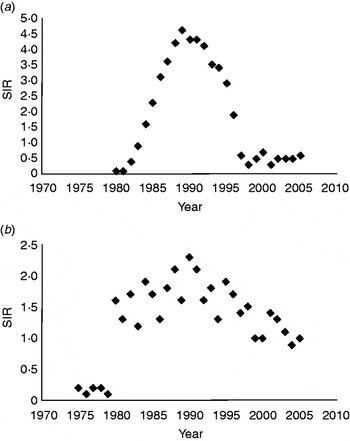
Fig. 1. Trends and standardized incidence ratio (SIR)* for (a) AIDS-related Kaposi's sarcoma and (b) classic Kaposi's sarcoma in the US population from 1980 to 2005 (SEER-9). (* SIR per 100 000 people per year, age-standardized to the 2000 US population. Relative changes in rate across nine SEER registries shown on a linear scale; P < 0·05.) SEER, Surveillance Epidemiology and End Results.
Of the 11 135 AIDS-related KS cases, 9356 were diagnosed from 1980 to 1995 and 1779 from 1996 to 2005. In AIDS-related KS, the age-adjusted SIR increased precipitously from 0·1 in 1980 to >4 between 1988 and 1992 (P = 0·05), peaking at 4·6 in 1989 (Fig. 1a). Since 1992, a steady decline in AIDS-related KS incidence was observed, with SIR decreasing to 0·3 in 1998 (P = 0·05) and reaching a plateau of 0·5 between 1999 and 2005.
Of the 871 classic KS cases, 121 were diagnosed between 1975 and 1979, 469 from 1980 to 1995, and 281 from 1996 to 2005. Between 1975 and 1979, the age-adjusted SIR of classic KS remained at a steady rate of about 0·2 (Fig. 1b). During the 1980s and 1990s, SIR for classic KS fluctuated between 1·2 and 2·3 (P = 0·68), with a mean of 1·7. In the early 2000s, the mean SIR for classic KS remained stable at 1·0.
Geographical variation in incidence of AIDS-related and classic KS
Of the AIDS-related KS cases between 1980 and 2005, the age-adjusted SIR appear to be highest in metropolitan areas (Table 2). Specifically, in AIDS-related KS cases in San Francisco, the rates rose from 0·2 (95% CI 0·1–0·4) in 1980 to 17·2 (95% CI 15·8–18·6) in 1990 and then declined to 1·6 (95% CI 1·3–2·1) by 1998. The age-adjusted SIR for other regions in USA included 2·2 in Atlanta, 1·3 in Hawaii and Puget Sound/Seattle, 0·3 in Utah, and 0·1 in Iowa.
Table 2. SIR of AIDS-related and classic KS based on geographical distribution from 1980 to 2005
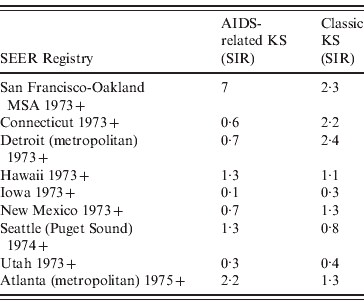
SIR, Standardized incidence ratio; MSA, metropolitan statistical area; KS, Kaposi's sarcoma; SEER, Surveillance Epidemiology and End Results.
SIR are per 100 000 and age-adjusted to the 2000 US Standard Population (19 age groups – Census P25–1130 ), P < 0·05.
While the overall SIR for classic KS was lower than that of AIDS-related KS during 1980–2005, in general, the metropolitan regions exhibited higher classic KS incidence compared to the more rural areas. For example, the SIR for classic KS in San Francisco was 2·0 (95% CI 0·6–4·7) in 1980 and 2·6 (95% CI 1·1–5·2) in 1990. The SIR of classic KS from 1980 to 2005 in other regions were 2·4 in Detroit, 2·2 in Connecticut, 1·3 in New Mexico and Atlanta, 1·1 in Hawaii, 0·8 in Puget Sound/Seattle, 0·4 in Utah, and 0·3 in Iowa (Fig. 2).
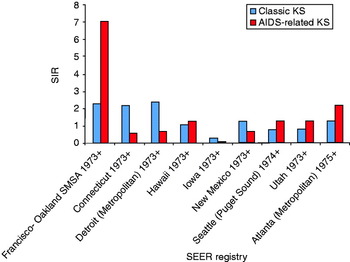
Fig. 2 [colour online]. Comparison of the standardized incidence ratio (SIR) of classic and AIDS-related Kaposi's sarcoma (KS) based on geographical distribution in the US population from 1980 to 2005. Distribution based on the SEER-9 registries. SEER, Surveillance Epidemiology and End Results.
Survival among AIDS-related and classic KS patients in the USA
The 5-year, age-adjusted survival rates for patients with AIDS-related KS were 12·1% (95% CI 11·4–12·8) during 1980–1995 and 54·0% (95% CI 51·4–56·8) during 1996–2005; the difference in these survival rates between the two periods was significant (P = 0·05). In comparison, the 5-year, age-adjusted relative survival rates for patients with classic KS from 1975 to 2005 was 85·0% (95% CI 68·1–93·3) during 1975–1979, 75·7% (95% CI 66·1–82·9) during 1980–1995, and 88·6% (95% CI 77·4–94·6) during 1996–2005 (Table 3).
Table 3. Age-adjusted 5-year survival rates for patients with AIDS-related and classic KS from 1975 to 2005
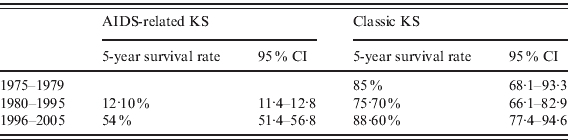
KS, Kaposi's sarcoma; CI, confidence interval.
DISCUSSION
The overall SIR of AIDS-related KS during 1980–2005 was not significantly higher than that of classic KS from 1975 to 2005 (P = 0·78). However, over a 25-year period from 1980 to 2005 in the USA, significant changes in incidence and survival rates resulted in a dynamic annual-level SIR trend in AIDS-related KS while classic KS rates have remained relatively stable. Therefore, we urge caution when comparing the overall SIR of each form of KS as an in-depth examination of annual SIR trends of each form of KS is also warranted.
The rise in KS incidence during the AIDS epidemic in the 1980s and 1990s was accountable for by the transient surge in overall KS incidence in the USA. Patients who were infected with the HIV virus were likely to also harbour HHV8. Of the AIDS-related KS cases, 98·8% were men [Reference Greenblatt22]. Our results revealed a disproportionately increased incidence of AIDS-related KS in metropolitan areas, such as San Francisco compared to the rural states of Iowa and Utah.
The timing of the peak incidence of AIDS-related KS varies within the literature. Some authors have suggested a peak incidence in AIDS-related KS in the early to mid-1980s with an ensuing decline in incidence from this time point to the late 1990s when the incidence stabilized [Reference Engels18]. However, our results demonstrat an increasing incidence during the mid-1980s with a peak incidence occurring in the late 1980s or early 1990s. This later occurring peak coincides with results from other epidemiological studies which describe a peak incidence that occurs during 1987–1991 [Reference Eltom11]. Furthermore, some authors place the peak incidence in AIDS-related KS as late as 1992 [Reference Shiels20]. The timing of the peak in incidence is a critical concept since the peak marks the beginning of the initial decline in AIDS-related KS incidence and improvement in survival. Therefore, accurately identifying when the peak occurred may allow for sociological, historical, and public health inferences to be drawn.
While our results reveal a later peaking incidence than reported by some authors, our data corroborate the finding that AIDS-related KS incidence declined in the mid-1990s in the USA [Reference Eltom11, Reference Engels18]. This subsequent sharp decline during the mid- to late-1990s paralleled the introduction of highly active antiretroviral therapy (HAART) and improved therapies for other HIV-related complications [Reference Eltom11, Reference Mocroft16, Reference Engels18]. Similarly, our results reveal that the previously described stabilization of the incidence of AIDS-related KS which occurred after 1996 has now remained for nearly a decade [Reference Engels18].
Moreover, the improvement of survival rates for patients with AIDS-related KS from 12·1% during 1980–1995 to 54·0% during 1996–2005 (P = 0·05) highly correlates with the advent of HAART in 1996. The improved survival rates of patients with KS probably reflect public health efforts, changes in HHV8 prevalence, and advances in treatment of HIV and its complications.
Compared to AIDS-related KS, the epidemiology of classic KS has remained relatively stable, although incidence of classic KS during 1980–2005 is slightly greater than that during the late 1970s. This modest increase may be attributed to a variety of factors. It is most likely that heightened awareness of KS following the advent of the AIDS epidemic resulted in the increased detection and reporting of KS cases [Reference Hengge23]. Alternatively, iatrogenic immunosuppression may have occurred more readily during this time predisposing more individuals to potentially developing KS.
Despite sharing a common viral agent (HHV8), the survival rates for patients with classic KS have also remained stable over the past three decades. Such a stable survival rate in patients with classic KS is not surprising. While improvements in antiretroviral therapies in the 1990s improved the overall survival in AIDS patients and thereby improved the survival of patients with AIDS-related KS, no significant improvement in treatment options in patients with classic KS occurred during this period. Treatment options for classic KS range from local treatments such as surgery, radiation, intralesional injections, or cryotherapy to systemic options. Unfortunately, the literature evaluating the efficacy of treatment options for classic KS is weak with most sources consisting of retrospective reviews or case reports [Reference Krown, Singh and Basow24].
As a registry-based study, our investigation has several potential limitations. First, the communities included in the SEER registries may not be representative of the entire US population. Second, the potential for misclassification bias exists. Utilizing previously described criteria [Reference Williams19], the 121 cases of KS revealed in the pre-AIDS era from 1975 to 1979 were automatically considered classic KS. However, applying the aforementioned criteria used to delineate AIDS-related from classic KS during 1980–2005 to the pre-AIDS cases would have changed the resulting incidence rates. Classifying those individuals in the 121 cases from 1975 to 1979 who were aged <70 years as ‘AIDS-related’ cases would have altered the incidence, albeit slightly, for overall classic and AIDS-related rates. Similarly, for the 1980–2005 period, it is possible that, according to our criteria, cases of KS were misclassified as AIDS-related when an older patient who has not yet reached age 70 years developed the classic form. While only 4–8% of cases of classic KS occur in individuals aged <50 years [Reference Di Lorenzo25], the higher threshold of age 70 years could possibly result in incorrectly identifying some individuals in their 50s and 60s as possessing AIDS-related KS. Last, as with any registry study, we need to consider the potential for under-reporting of KS cases.
In conclusion, while some sources suggests that peak incidence of AIDS-related KS occurred in the early to mid-1980s and then subsequently declined throughout the 1980s and 1990s [Reference Engels18], our results support an increasing SIR of AIDS-related KS which reached its maximum in the late 1980s or early 1990s. Our data corroborate the steady decline in AIDS-related KS incidence in the mid-1990s. This decrease in AIDS-related KS incidence and increased survival rates are probably attributable to the advent of effective antiretroviral therapy. Furthermore, our results demonstrate the stabilization in this AIDS-related SIR after 1996 has extended for nearly a decade. Finally, the SIR for classic KS remained relatively stable from 1975 to 2005 compared to the SIR for AIDS-related KS. Without significant improvement in the treatment options for classic KS, the survival rate has likewise remained stable.
DECLARATION OF INTEREST
None.







