INTRODUCTION
Pertussis is a vaccine-preventable disease caused by the Gram-negative bacillus Bordetella pertussis. Pertussis is a coughing illness that is typically characterized by paroxysms of coughing that may be associated with vomiting, apnoea or cough with a distinctive whoop. Pertussis in young children can result in serious illness or death, particularly in those too young to be vaccinated. Although many developed countries have long established vaccination programmes for pertussis, it remains one of the most commonly reported vaccine-preventable diseases [Reference Wood and McIntyre1].
In many countries, pertussis is a notifiable condition and health practitioners and laboratories are required by law to notify health departments of all diagnosed cases. This information is used to follow-up cases for control measures (e.g. prophylaxis of contacts) and to monitor the epidemiology of pertussis.
Pertussis can be very severe in young or unvaccinated children and may result in presentation to an emergency department (ED) or admission to hospital, where the event may be recorded in the hospital or ED administrative database. Hospital administrative data have been shown to be a useful source of information on the epidemiology of pertussis [Reference Bonacruz-Kazzi2]. Pertussis diagnoses coded in hospital records have been found to be of high quality and relatively consistent over time, and may be less influenced by testing or reporting biases than notifiable disease data [Reference Bonacruz-Kazzi2]. In recent years, ED presentation data have been used for syndromic surveillance of conditions such as influenza-like illness, and have been shown to correlate well with increases in notifications of influenza [Reference Begier3, Reference Fleischauer4]. However, ED data have been little used for monitoring the epidemiology of specific infections such as pertussis, due in part to variability in the collection and quality of disease-specific diagnoses or chief complaints in these data.
Data linkage presents an opportunity to overcome or minimize the limitations of underreporting and variability in routinely collected data. With improvements in computing power, linkage of very large administrative datasets is becoming increasingly common. Probabilistic methods have been developed that enable linkage in the absence of unique personal identifiers [Reference Jaro5].
Previous studies of pertussis using linked data have predominately focused on aspects of pertussis vaccination such as vaccine safety [Reference Gold6–Reference Aaby8], vaccine uptake [Reference Santoli9, Reference Schulte10], and the validity of linked data for vaccine effectiveness studies [Reference Mahon11, Reference Hviid12]. Fewer studies have examined risk factors for infection [Reference Haberling13–Reference Liu15] and long-term consequences of infection [Reference Bager16]. We could find no studies which linked data on pertussis from statutory notifications to coded data on hospitalizations and ED presentations.
Estimates of the burden on hospitals are important for calculating the cost benefit of vaccination programmes and may also be useful for health service planning, particularly during epidemic periods. The lack of data on the burden on EDs means that the costs associated with ED presentations are rarely included in cost-benefit analyses.
The objectives of our study were to (a) examine hospital and ED records that linked to notified pertussis cases; (b) determine the time from notification to hospital admission or ED presentation with a coded diagnosis of pertussis; (c) describe hospital admissions and ED presentations that occur around the time of pertussis notification; and (d) estimate the additional burden of pertussis on hospitals and EDs ascertained by using linkage of administrative records.
METHODS
Setting
Our study used whole-of-population information for the state of New South Wales (NSW), Australia. NSW is Australia's most populous state, with a total resident population of 6 549 177 in 2006 [17], of whom 1298917 (19·8%) were aged <15 years.
Data sources and data linkage
We used five routinely collected population-based datasets in NSW: the Perinatal Data Collection, Notifiable Conditions Information Management System, Admitted Patient Data Collection, Emergency Department Data Collection, and death registrations. These were linked using probabilistic methods by the NSW Centre for Health Record Linkage (CHeReL) [18], according to a ‘best practice protocol’ for preserving individual privacy [Reference Kelman and Bass19].
The Perinatal Data Collection includes records for all births from 1 January 1994 of babies weighing at least 400 g or of at least 20 weeks' gestation. The records contain demographic details of the mother and details of the pregnancy and birth.
The Notifiable Conditions Information Management System contains records of notifiable diseases reported under the NSW Public Health Act 1991 from 1 January 1993. Information available on the records included data about the notification (who and when), laboratory confirmation (specimens, type and dates) and patient outcome (hospitalization, death). A calculated onset date was available which is defined as the earliest of notification, patient reported onset or specimen collection dates.
The Admitted Patient Data Collection contains demographic, administrative, diagnostic and procedural information from all NSW public and private hospitals and day procedure centres. Diagnoses and procedures for each admission are coded according to ICD-10-AM [20]. Records were available from 1 July 2000.
The Emergency Department Data Collection captures an estimated 83% of presentations to EDs in NSW public hospitals [18]. Information on patient demographics and provisional diagnoses is available; diagnoses are coded using ICD-9 [21] ICD-10-AM [20] or SNOMED-CT [22] classification schemes. Records were available from 1 January 2005.
We used death records from two sources: NSW Registry of Births, Deaths and Marriages (RBDM) death registrations and Australian Bureau of Statistics (ABS) mortality records. NSW RBDM records contain only fact of death information and include data from 1 January 1994. ABS records contain information on the cause of death coded according to ICD-10 [23] and include data from 1 January 1994.
We used records from all datasets up until 31 December 2008, with the exception of the ABS mortality records which were available only up until 31 December 2007 due to a delay in the release of data by the ABS.
Study population and definitions
We included all children born between 1 January 1994 and 31 December 2008 to NSW resident mothers where a record of the birth was included in the Perinatal Data Collection. Children were excluded if they had duplicate perinatal records (n = 225); were stillborn or died before 1 January 2005 (n = 14 054); or had a pertussis notification record where the notification date and earliest of specimen collection, patient reported onset or hospital admission date were more than 6 months apart (n = 7).
Pertussis notifications included cases with definitive laboratory evidence; or laboratory suggestive evidence together with clinical evidence; or clinical evidence together with an established epidemiological link to a confirmed case with laboratory evidence. Laboratory definitive evidence required isolation of B. pertussis from a clinical specimen or detection of B. pertussis by nucleic acid testing. Laboratory suggestive evidence required seroconversion or significant increase in antibody (IgA or IgG) level or a ⩾fourfold rise in titre to B. pertussis whole cell (IgA only) or B. pertussis specific antigen (in absence of recent vaccination) or a single high IgA titre to whole cells or detection of B. pertussis antigen by immunofluorescence assay. Clinical evidence required a coughing illness lasting ⩾2 weeks or paroxysms of coughing or inspiratory whoop or post-tussive vomiting [24].
The pertussis diagnosis date was defined as either the specimen collection date as recorded in the notification records or, where the specimen collection date was missing, the earliest date recorded on the notification. The method of diagnosis was determined from the specimen type and laboratory confirmation status as recorded on the notification record: where the specimen type was aspirate or swab, the method of diagnosis was classified as ‘PCR/culture’; where the specimen type was serum, the method of diagnosis was classified as ‘serology’; where the laboratory confirmation status was ‘no’, the method of diagnosis was classified as ‘clinical’; and for the remaining records, method of diagnosis was classified as ‘unknown’ (n = 81).
The Admitted Patient Data Collection records contain a primary diagnosis and up to 54 additional diagnostic codes per record and may include multiple records for an admission if the patient was transferred during their stay. All records relating to one hospital stay were grouped, and the primary diagnosis recorded on the last record in the set was used as the primary diagnosis for the admission.
The Admitted Patient Data Collection records were classified as having a coded diagnosis of ‘pertussis' if any diagnosis field had an ICD-10-AM code of A37. Emergency Department Data Collection records were classified as having a coded diagnosis of ‘pertussis' if the provisional diagnosis had an ICD-10-AM code of A37, ICD-9 code of 033 or if the SNOMED-CT code description contained ‘whooping cough’ or ‘pertussis'.
Statistical analysis
We first counted the number of records with a coded diagnosis of pertussis from each data source for each child during the period 1 January 2005 to 31 December 2008 and calculated incidence rates for the first pertussis diagnosis from each source (notification, hospital admission or ED presentation). Person-time at risk began at the latest of either 1 January 2005 or date of birth and ended at the earliest of pertussis event, death or 31 December 2008. Person-time at risk was apportioned to four age groups: 0 to <6 months, 6 to <24 months, 2 to <5 years and 5 to <15 years.
Next, we changed our focus from children to individual records of hospital admissions and ED presentations. We selected all hospital admissions and ED presentations and determined if they linked to a pertussis notification. We then examined the distribution of time from notification to admission/presentation. To determine if there were any particular diagnoses that were more likely to be recorded on admissions/presentations that occurred around the time of a pertussis notification, but did not have a coded diagnosis of pertussis, we counted the frequency of each primary diagnosis occurring on admissions/presentations within a time window of ±7 days from the notification date and compared this with the frequency of the primary diagnosis in all other admissions/presentations. For diagnoses with at least five admissions or presentations, the relative risk of having an admission/presentation for the specific primary diagnosis within ±7 days from notification vs. having such an admission/presentation outside of this period, was calculated using log-linked binomial regression models adjusted for age and year of admission/presentation. Age group at admission was included as a categorical variable and year of admission as a continuous variable.
We then returned our focus to children and recalculated the incidence rates incorporating all of the additional hospital admissions and ED presentations occurring within ±7 days from notification, as well as those that had a coded diagnosis of pertussis. The proportion of children with a pertussis notification that were admitted to hospital or presented to an ED was calculated using each definition (coded diagnosis of pertussis vs. occurred within ±7 days from notification or had a coded diagnosis of pertussis) and compared using McNemar's test.
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., USA). An area-proportional Venn diagram was constructed using BioVenn [Reference Hulsen, de Vlieg and Alkema25].
Ethical approval
This study was approved by the NSW Population and Health Services Research Ethics Committee.
RESULTS
After exclusions, a total of 1 304 876 children were included in the study. Over the period 1 January 2005 to 31 December 2008, 3006 children had a notification for pertussis. In the cohort, 455 children had 492 hospital admissions with a diagnosis of pertussis recorded in any field, of whom 323 (71%) also had a notification for pertussis. A total of 644 children had 683 ED presentations coded as pertussis, of whom 265 (41%) also had a notification and 165 (26%) also had a hospital admission for pertussis (Fig. 1).
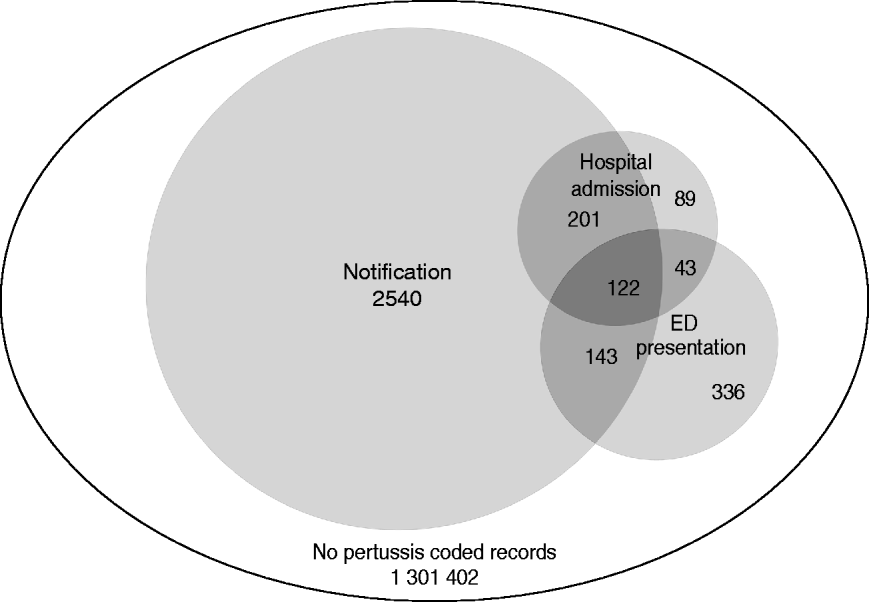
Fig. 1. Number of children with a pertussis notification, hospital admission or emergency department (ED) presentation record with a pertussis code (ICD-9, ICD-10 or SNOMED) in any diagnosis field from 2005 to 2008 by type of record. The area of circles is proportional to the number of notifications, hospital admissions and ED presentations.
Notifications
Of 3006 children with a pertussis notification, 2768 (92%) had a recorded laboratory test of which 2256 (75%) were PCR or culture confirmed. The proportion of notifications that were PCR/culture confirmed increased over time from 49% in 2005, to 68% in 2006, 69% in 2007 and 80% in 2008. The year 2008 also marked the beginning of a new epidemic cycle, with 2241 notifications compared to 226–297 per year in 2005–2007. Children aged <6 months had the highest notification rate (Table 1).
Table 1. Pertussis notification, hospital admission and emergency department (ED) presentation rate per 100 000 person-years, 1 January 2005 to 31 December 2008
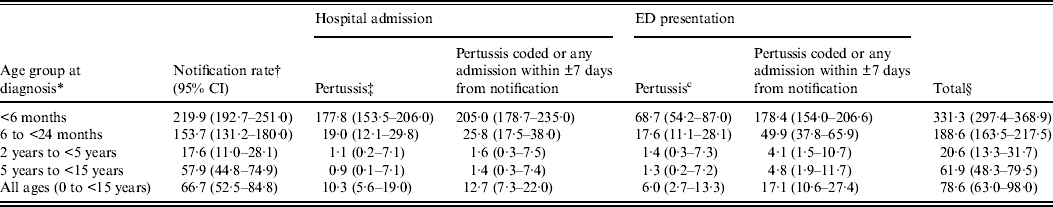
CI, Confidence interval.
* Notification date is defined as the specimen collection date or where missing, the earliest date recorded on the notification record.
† Rate: numerator is the total number of children with pertussis; denominator is person-time at risk.
‡ Pertussis: hospital or ED record contains a diagnostic code for pertussis (ICD-10-AM A37 or equivalent).
§ Notification, hospital admission or ED presentation coded as pertussis.
Hospital admissions
The codes recorded for the 492 pertussis hospital admissions were as follows: B. pertussis (ICD-10-AM A37·0) in 237 (48%); Bordetella, species unspecified (A37·9) in 252 (51%); and B. parapertussis (A37·1) in three (0·6%). Pertussis was the primary diagnoses in 393 (80%) of these admissions with the two other most common primary diagnoses being acute bronchiolitis 39 (8%) and other respiratory illnesses 27 (5%).
Of children with both a notification and a hospital admission coded as pertussis (n = 323), the median time from notification to hospital admission was 0 days [range −220 to 111, interquartile range (IQR) −1 to 3]. The majority (83%) of pertussis-coded admissions occurred within ±7 days from notification, and there was a similar distribution for admissions that did not have a pertussis diagnostic code (Fig. 2); this finding remained unchanged (83%) when restricted to notifications which were PCR or culture confirmed.
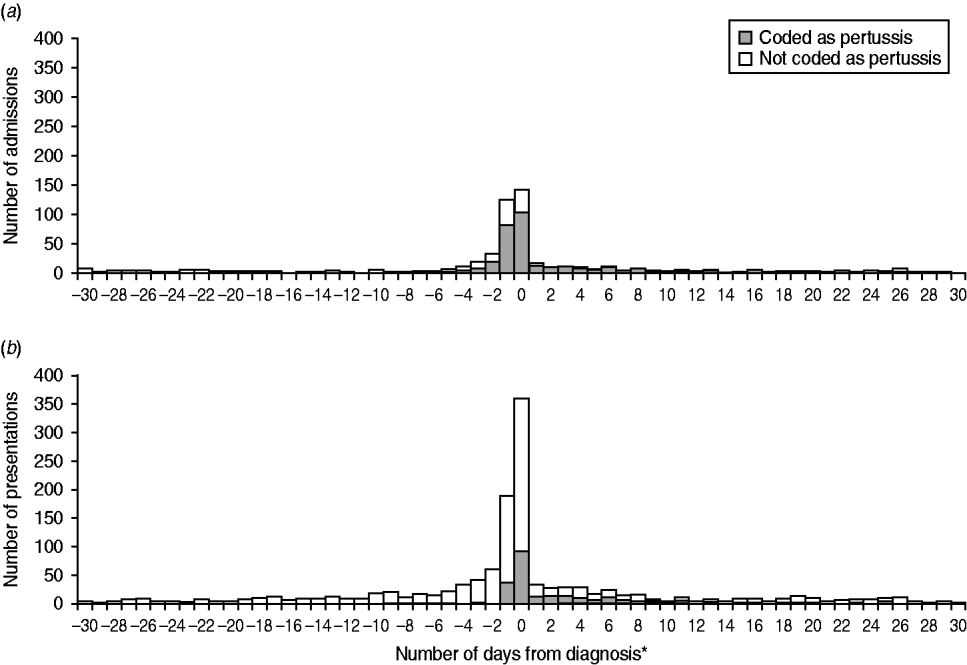
Fig. 2. Number of (a) hospital admissions and (b) emergency department presentations that linked to a pertussis notification in the 30 days prior to and following notification. * Diagnosis date is defined as the specimen collection date or where this is missing, the earliest date recorded on the notification record is used.
Of the 3006 children with a pertussis notification, 140 admissions in 127 children occurred within ±7 days of the notification but pertussis was not listed in any of the 55 diagnostic fields. Primary diagnoses in these additional 140 admissions were most commonly acute bronchiolitis (53, 38%) and other respiratory illness (41, 29%).
Admissions occurring within ±7 days from a child's pertussis notification were 16 times more likely than other admissions to have a primary diagnosis of acute bronchiolitis [adjusted for age and year of admission: relative risk (RR) 15·8, 95% CI 12·6–19·8, P < 0·0001] and eight times more likely to have a primary diagnosis of acute upper respiratory tract infection (adjusted RR 8·0, 95% CI 4·5–14·0, P < 0·0001) (Table 2).
Table 2. Primary diagnoses recorded on non-pertussis-coded hospital admissions and emergency department (ED) presentations occurring within ±7 days of a pertussis notification compared to admissions occurring at other times or in children who did not have a pertussis notification
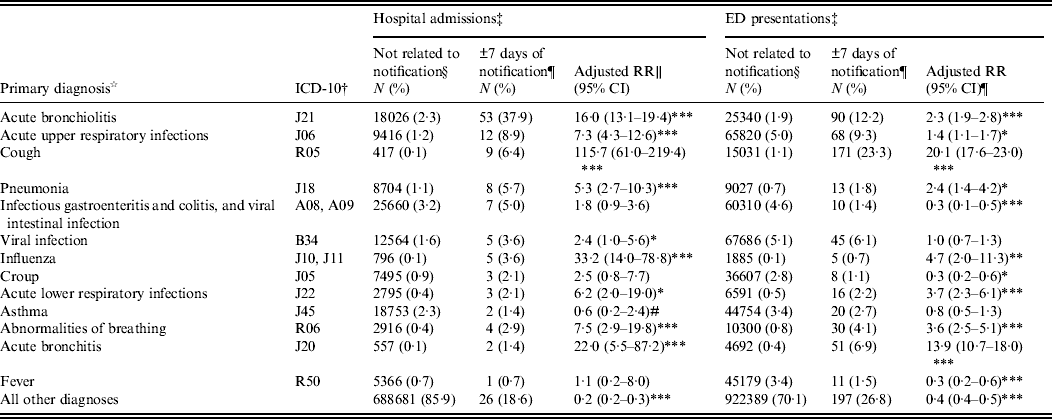
RR, Relative risk; CI, confidence interval.
Primary diagnoses listed are those where at least five admissions with this diagnosis occurred in the 7 days either side of a pertussis notification. All admissions with a diagnosis of pertussis in any diagnosis field have been excluded.
† ICD-10-AM codes have been truncated to the first three characters. All admissions with a primary diagnosis beginning with these three characters are included. Equivalent ICD-9 and SNOMED-CT codes have been used for ED presentations where ICD-10-AM codes are not available.
‡ Where a child had more than one admission or ED presentation, each separate admission/presentation is included in this analysis as a separate event.
§ Admissions not related to notification are those that occurred more than 7 days before or after diagnosis as determined from the notification record and those that occurred in children that did not have a notification for pertussis.
¶ Admissions that occurred within ±7 days from notification, where notification is the specimen collection date or next earliest date recorded on the notification record.
∥ Adjusted relative risks calculated using log-linked binomial regression model adjusted for age group and year of admission.
# Unable to estimate adjusted RR, crude RR reported.
*P < 0·05, ** P < 0·001, *** P < 0·0001.
Age-specific and overall rates of hospital admission increased when all these additional ‘window period’ admissions were added to those with a coded diagnosis of pertussis (Table 1). The proportion of children classified as having a hospital admission significantly increased in all age groups (McNemar's test P < 0·0001) except in those aged 2–5 years (McNemar's test P = 0·063, Table 3).
Table 3. Percentage of children with a pertussis notification who had a linked hospital or emergency department (ED) presentation in NSW, Australia, 1 January 2005–31 December 2008
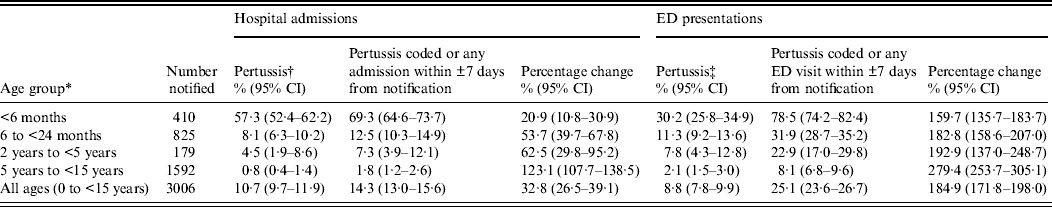
CI, Confidence interval.
* Age was calculated based on the age at notification, where the notification date was either the specimen collection date or where this was missing, the earliest date recorded on the notification record.
† The hospital admission record included an ICD-10-AM code for pertussis (A37) in any of the 55 diagnoses fields.
‡ The ED record included an ICD-10-AM, ICD-9 or SNOMED code for pertussis in the provisional diagnosis field.
ED presentations
Almost all ED presentations with a diagnosis of pertussis were coded as whooping cough, unspecified organism (ICD-10-AM A37·9), 670 (98%) with only 13 (2%) coded as B. pertussis (ICD-10-AM A37·0). Of children with both a notification and ED presentation for pertussis, the median time from specimen collection to ED presentation was 0 days (IQR 0–5 days). Although the majority (78%) of pertussis-coded ED presentations occurred within ±7 days from notification, many ED presentations that did not have a pertussis diagnosis also occurred around the time of notification (Fig. 2) and this finding persisted when restricted to those linked to a PCR-/culture-confirmed notification (82%).
Examination of linked ED presentation records for the 3006 children with a pertussis notification from 1 January 2005 to 31 December 2008 identified 735 ED presentations in 567 children that occurred within ±7 days of a child's pertussis notification that did not have a coded diagnosis of pertussis. The most common specific diagnoses recorded for these presentations were cough (171, 23%), acute bronchiolitis (90, 12%), upper respiratory infection (68, 9%), bronchitis (51, 7%) and viral infection of unspecified site (45, 6%).
These presentations not coded as pertussis were significantly more likely than other presentations to be coded as acute bronchiolitis (adjusted for age and year of presentation, RR 2·3, 95% CI 1·9–2·8, P < 0·0001); cough (RR 20·1, 95% CI 17·6–23·0); influenza (RR 4·7, 95% CI 2·0–11·3, P < 0·0001); abnormalities of breathing (RR 3·6, 95% CI 2·5–5·1, P < 0·0001) and acute bronchitis (RR 13·9, 95% CI 10·7–18·0, P < 0·0001), and significantly less likely to be coded as fever (RR 0·3, 95% CI 0·2–0·6, P = 0·0001) (Table 2).
Age-specific and overall rates of ED presentation increased when the ±7 days window period presentations were added (Table 1) and in children with a pertussis notification, the proportion of children classified as having an ED presentation significantly increased in all age groups (McNemar's test P < 0·0001, Table 3).
For both hospitalizations and ED presentations we also looked at a wider time window (−7 to +14 days) and (−14 to +21 days) from notification but findings did not differ substantially (results not show).
Deaths
There were no deaths with a coded cause of death of pertussis in the cohort in the period 2005–2007. Linked data identified two children with a pertussis notification who died, but this was 298 and 317 days following onset of pertussis.
DISCUSSION
Using linked data allowed us to identify additional hospital admissions and ED presentations that occurred around the same time as a pertussis notification and these were significantly more likely than presentations occurring in other time windows to have diagnoses consistent with pertussis, such as acute bronchiolitis, acute respiratory infection and cough. This suggests that they were highly likely to be pertussis-related and estimates of the proportion of children with pertussis admitted to hospital or seen at an ED increased substantially when these additional events were included.
Our findings are supported by those of a recent study that used linked laboratory reports and hospital data to determine the frequency of respiratory pathogens in hospital admissions for acute lower respiratory infection in Western Australia. The authors reported that B. pertussis was identified in a high proportion of hospital admissions coded as bronchiolitis (17%), pneumonia (12%) or influenza (22%) where testing was requested and commented that as testing for B. pertussis is not routine, these cases may represent atypical clinical presentation or asymptomatic infection [Reference Moore26].
Few studies have published rates of ED presentation in children with pertussis and surveillance reports in NSW do not routinely report ED presentations. A survey of households in the USA where a case of pertussis had been notified to a local department of health reported that four (8%) out of 52 children aged 0–9 years with a pertussis notification presented to an ED, and five (10%) required hospitalization [Reference Lee and Pichichero27]. These rates were similar to those in our study in terms of admissions and presentations with a coded diagnosis of pertussis (9% presented to ED, 11% admitted to hospital), but substantially lower than when those that occurred in our ±7-day window period (25% and 14%, respectively) were included. Not surprisingly, our data showed that the proportion presenting to an ED was highest in children aged <6 months. The increase in the proportion hospitalized or presenting to an ED was greatest in the older age groups, particularly those aged 5 to <15 years. This would suggest pertussis in this age group continues to be less well recognized in hospitals and EDs or may take longer to diagnose, as previously reported [Reference Cagney28, Reference Deeks29].
A relatively large proportion of hospital admissions (29%) and ED presentations (59%) coded as pertussis were not notified, consistent with a previous Australian study [Reference Bonacruz-Kazzi2] that found only 56% of coded hospitalizations were notified, largely limited to laboratory-confirmed cases for which reporting is a legislative requirement. Since that study was conducted (1997–1999), the use of PCR testing has increased substantially in Australia [Reference Spokes, Quinn and McAnulty30] and because it is more sensitive than the culture or serological methods used in Australia [Reference Loeffelholz31], it is likely to account for the smaller proportion of non-notified pertussis hospital admissions found in our study (29%). Although previous studies have not reported the proportion of non-notified ED presentations for pertussis, our findings are not surprising as previous studies have highlighted a number of factors which limit clinical reporting of notifiable diseases [Reference Backer, Bissell and Vugia32–Reference Turnberg, Daniell and Duchin35]. Of particular relevance for ED settings is the limited access to laboratory results, lack of time and the provisional nature of ED diagnoses.
The key strength of our study was the use of linked administrative data as it allowed a more complete assessment of the healthcare burden of pertussis without relying on one source of diagnostic information. Measuring the ED burden is of particular interest because it provides a more complete estimate of the total morbidity and costs and also has potential implications for infection control in ED settings. Additional studies using linked data could investigate whether there is evidence of transmission in ED settings.
Our study had several limitations. Admissions and presentations in NSW residents who attend hospitals and EDs in neighbouring states are not included in the datasets and we were unable to identify children born in NSW who had moved to other jurisdictions during the study period. The ED data we used included 83% of ED presentations for the NSW population, with smaller rural EDs excluded. Therefore, we probably did not capture all pertussis-related presentations in the study cohort. Similarly, we were not able to validate diagnoses recorded in hospital or ED records, so some may have been misclassified. However, our comparison of the primary diagnosis on hospital admissions and ED presentations occurring within ±7 days of a pertussis notification supported the notion that most of these events in notified cases were pertussis-related. We demonstrated that the distribution of primary diagnoses in admissions and presentations was very different from the ‘background’ of admissions and presentations in the cohort, with those occurring within ±7 days of a notification being much more likely to have diagnoses consistent with signs or symptoms of pertussis than other admissions.
As we did not restrict admissions/presentations counted as occurring within ±7 days from notification to specific diagnoses, we may have overestimated the incidence of pertussis-related hospital admissions and ED presentations. Conversely, as only 83% of hospital admissions and 78% of ED presentations with a coded diagnosis of pertussis occurred within ±7 days from notification, our ±7-day window for identifying non-coded events may have been too restrictive, thereby underestimating the incidence of pertussis-related events. Using the diagnosis date to calculate this time period also influenced our results and using other dates such as notification date may reduce the number of additional events identified, because the distribution of time from notification to hospital admission/ED presentation was more variable. Finally, seroprevalence studies indicate that a large number of pertussis infections go unreported and/or undiagnosed [Reference Mark and Granstrom36]; therefore, this may also mean that our pertussis-related hospital admission and ED presentation rates were underestimated.Accurate estimates of the cost of pertussis on the health system are important as they provide the basis for decisions about the cost-effectiveness of proposed vaccination strategies. Although many studies conduct a sensitivity analysis to determine the effect of variations in the estimates of incidence of pertussis, most have not conducted a sensitivity analysis around the variation in the rates of hospitalizations or ED presentations. Our findings suggest that the costs associated with ED and hospital care of children with pertussis may have been underestimated in cost-effectiveness studies.
With the increasing availability of electronic vaccination registries, it will become possible to conduct large population-based vaccine-effectiveness studies using administrative data [Reference Mahon11, Reference Skowronski37]. The findings of our study have implications for this type of vaccine-effectiveness study as we have shown that a substantial proportion of hospital admissions and, in particular, ED presentations are likely to be missed if researchers rely on coded diagnoses of pertussis alone.
Linked administrative data allowed us to identify a substantial number of hospital admissions and ED presentations that occurred around the time of a pertussis notification in children that were likely to be pertussis-related but were not coded as such. This enabled us to make more comprehensive estimates of the health system burden of notifiable diseases, and we would encourage researchers to undertake similar studies in other settings.
ACKNOWLEDGEMENTS
This work was supported by the National Health and Medical Research Council under the Outcomes, Services and Policy for the Reproductive and Early Years (OSPREY) project (grant no. 573122). B.L. is supported by a fellowship from the National Health and Medical Research Council. The authors acknowledge the NSW Ministry of Health for providing the data and the Centre for Health Record Linkage (CHeReL) for performing the data linkage.
DECLARATION OF INTEREST
None.






