INTRODUCTION
In the face of annual influenza epidemics, a potential avian influenza pandemic, fluctuating vaccine supplies, and uncertainty about antiviral availability, the need for influenza surveillance is great. Influenza surveillance systems can (1) identify the presence of influenza and track trends in disease activity; (2) characterize viral isolates to dictate future vaccine composition, identify the emergence of new strains, and detect changes in antiviral resistance; (3) assess the burden of disease in different age and risk groups; and (4) estimate vaccine impact [Reference Hilleman1–Reference Iwane5]. Current influenza surveillance systems in the United States have been designed primarily to address the first two goals. Estimating disease burden, has been done primarily by modelling hospital discharge data [Reference Thompson6].
Although the identification of all cases of influenza or an unbiased sample of cases without regard to vaccination status would be ideal to quantify disease burden and to assess vaccine effectiveness, the main constraint is the time and expense of accurately enumerating all influenza-associated events. An alternative attractive approach is to estimate the disease burden using data from two or more surveillance systems, neither of which needs to be comprehensive. Decades ago, capture–recapture methods emerged as an adaptation of techniques applied by wildlife researchers. When appropriately employed, these methods can provide a more accurate, quick and relatively inexpensive estimation of the disease burden [Reference Goldman7–Reference Laska10]. Capture–recapture techniques have been recently used to estimate the burden of influenza hospitalizations in children in Davidson County, Tennessee, during the moderately severe 2003–2004 influenza season [Reference Grijalva11]. That study also highlighted some specific strengths and limitations of current influenza surveillance systems. In the present study, we applied these techniques to three metropolitan counties in three regions in the United States during the 2004–2005 influenza season.
METHODS
The New Vaccine Surveillance Network (NVSN) was established in 1999 by the Centers for Disease Control and Prevention (CDC) to perform active surveillance for common respiratory viral infections and to assess the impact of new vaccines and vaccine policies in children aged <5 years in selected US counties. Using methodologies described earlier, the NVSN conducted active surveillance in Davidson County, Tennessee; Hamilton County, Ohio; and Monroe County, New York during the 2004–2005 influenza season [Reference Iwane5, Reference Grijalva11–Reference Poehling13]. County residents younger than 5 years, hospitalized with respiratory symptoms and/or fever were enrolled within 48 h of admission after parental/guardian informed consent. One or two hospitals in each county were included and accounted for at least 95% of all paediatric admissions for acute respiratory infections in the county. Surveillance was conducted 7 days per week. After informed consent, a questionnaire was administered to the parents and nasal and throat swabs were obtained for viral culture and RT–PCR performed in research laboratories. Newborns that never left the hospital, children whose parents refused enrolment, children ill for >14 days, and children with fever and neutropenia were excluded. A child was considered to have influenza if either the viral culture or duplicate RT–PCR assays were positive. The results of these research tests were not entered into the hospital chart and were not available to the clinicians [Reference Iwane5, Reference Poehling13].
The Emerging Infections Program (EIP), a cooperative effort of the CDC, state and local health departments, and academic health centres is a population-based surveillance network designed to assess the public health impact of emerging infections and to evaluate methods for surveillance, prevention, and control [14]. Starting in 2003, the EIP identified clinical laboratory-confirmed influenza-associated hospitalizations in patients aged <18 years in participating states. However, for the purposes of analysis in this study, only those children aged <5 years were included. Influenza cases were defined as hospitalized children with clinical laboratory evidence of influenza infection obtained as part of their usual medical care. Cases were actively identified through hospital laboratories, admissions departments, and infection control practitioners. Trained surveillance officers conducted medical chart reviews for all potential cases to confirm case eligibility and obtain demographic, clinical, and vaccination history information. In the EIP system, decisions about whether to test and which test to use were at the discretion of the practitioners responsible for the child's care. Commercially available rapid tests, viral culture, immunofluorescence antibody staining, RT–PCR, immunohistochemical staining and paired serology documenting a four-fold rise in influenza antibody titre were the diagnostic techniques accepted by the EIP system. A statement in the medical history that the child had a positive rapid test for influenza performed in the outpatient setting was also accepted. The EIP system excluded children who were hospitalized >14 days after they tested positive for influenza and children with a positive influenza test on a specimen collected >3 days after hospital admission.
For Davidson County, EIP was the only influenza surveillance system operating simultaneously with NVSN. In Hamilton County, laboratory-confirmed influenza-associated hospitalization surveillance, similar to that done by EIP was performed retrospectively by NVSN staff for quality control. In Monroe County, EIP surveillance was further supported by the Electronic Clinical Laboratory Reporting System (ECLRS), another system that used a methodology analogous to EIP, through which influenza laboratory data from the State of New York was aggregated into a single electronic system by the New York State Department of Health (NYSDOH) [15].
The present study was restricted to the 2004–2005 influenza season and included children aged <5 years who were residents of the three NVSN counties and for whom clinical laboratory surveillance was also in place. Cases identified by both surveillance systems were considered matched cases. The ascertainment of these matched cases was performed retrospectively at the end of the influenza season and was based on names, date of birth and admission and discharge dates. Data for final analyses were devoid of personal identifiers. This study was performed under an amendment of the NVSN research protocol approved by the Institutional Review Boards of the three participating research sites and the CDC.
The total number of children aged <5 years hospitalized with influenza during the surveillance period was estimated using Petersen's capture–recapture method [Reference Chao9]. Let N be the true number of patients and let ![]() be Peterson's estimate of N (Fig. 1). The first surveillance system (NVSN) captured n1 cases giving a capture rate of n1/N. The second system (EIP/EIP-like) captured n2 cases; including m2 cases that were already captured by the first system (recaptured or matched cases). The recapture rate was m2/n2. Since the probability of capture by one surveillance system was independent of capture by the other, the expected proportion of patients captured by the first system equalled the expected proportion of patients recaptured by the second. The corresponding observed rates, n1/N and m2/n2 should be similar and will differ only due to chance fluctuation in the capture rates. Equating these two observed rates gives Petersen's estimate of N which is
be Peterson's estimate of N (Fig. 1). The first surveillance system (NVSN) captured n1 cases giving a capture rate of n1/N. The second system (EIP/EIP-like) captured n2 cases; including m2 cases that were already captured by the first system (recaptured or matched cases). The recapture rate was m2/n2. Since the probability of capture by one surveillance system was independent of capture by the other, the expected proportion of patients captured by the first system equalled the expected proportion of patients recaptured by the second. The corresponding observed rates, n1/N and m2/n2 should be similar and will differ only due to chance fluctuation in the capture rates. Equating these two observed rates gives Petersen's estimate of N which is ![]() =(n1×n2)/m2. This population estimate is valid under the assumption that the probability of being captured by one system does not affect the probability of being captured by the other, that the study population remained approximately constant and without significant migration during the study period, and that ascertainment of influenza by both surveillance systems was valid [Reference Chao9, Reference Gjini16–Reference Wittes19].
=(n1×n2)/m2. This population estimate is valid under the assumption that the probability of being captured by one system does not affect the probability of being captured by the other, that the study population remained approximately constant and without significant migration during the study period, and that ascertainment of influenza by both surveillance systems was valid [Reference Chao9, Reference Gjini16–Reference Wittes19].

Fig. 1. Capture–recapture analysis using two independent sources. Peterson's estimator of N (total cases), ![]() =n1×n2/m2. Peterson's estimate implies that the estimated number of cases missed by both systems (
=n1×n2/m2. Peterson's estimate implies that the estimated number of cases missed by both systems (![]() ) equals (b×c)/(a); where b was the number of enrolled cases by EIP/EIP-like only, c was the number of enrolled cases by NVSN only, and a represented the number of matched cases (m2).
) equals (b×c)/(a); where b was the number of enrolled cases by EIP/EIP-like only, c was the number of enrolled cases by NVSN only, and a represented the number of matched cases (m2).
Confidence intervals for N were calculated using the likelihood-ratio support intervals [Reference Regal and Hook20]. In other words, the 95% confidence interval (CI) for N consisted of all population sizes for which the log-likelihood-ratio χ2 statistic was <3·84. The capture–recapture method was used to estimate the total number of influenza hospitalizations in children aged <5 years. Since NVSN attempted to test all children hospitalized with acute respiratory illness or fever, the age distribution derived from this system was considered likely to represent the true age distribution of cases. This age distribution was then applied to the capture–recapture estimated total of cases to derive age-specific estimates for children aged <6 months, 6–23 months, and 24–59 months.
Hospitalization rates were calculated by dividing the number of influenza hospitalizations by the county population denominator for children aged <5 years obtained from the 2000 US Census. The population of children aged <6 months was estimated as one-half the number aged <1 year. Sensitivities of each surveillance system were calculated by dividing the rates generated by each of these systems by the rate generated through the capture–recapture estimates. Analyses were performed using stata 8.2 software (Stata Corporation, College Station, TX, USA).
RESULTS
During the 2004–2005 influenza season, 98 influenza-related hospitalizations of children aged <5 years were identified in the study population (three counties). Of these, 83 children (85%) were identified by NVSN surveillance, and 48 (49%) were identified by EIP/EIP-like surveillance. Thirty-three (34%) children were identified by both systems (matched cases). The capture–recapture analysis estimated that there were 23 influenza hospitalizations that were undetected for an estimated overall number of 121 (95% CI 108–145) influenza hospitalizations (Fig. 2).

Fig. 2. Capture–recapture estimates. Influenza hospitalizations in children aged <5 years in three US counties, 2004–2005 influenza season.
Stratifying by age, influenza hospitalization rates were 43·8, 9·6 and 2/10 000 children aged <6 months, 6–23 months and 24–59 months, respectively (Table 1, Fig. 3). Taking the capture–recapture estimates as the reference, NVSN surveillance detected 69% of the influenza hospitalizations, whereas EIP/EIP-like surveillance detected 39%. This contrast was consistently observed for all age groups (Table 2). When compared to capture–recapture estimates, the sensitivities of NVSN surveillance in the three counties were 43%, 72%, and 77%. Similarly, the estimated sensitivities for EIP/EIP-like surveillance were 23%, 30%, and 56%.

Fig. 3. Influenza hospitalization rates in children aged <5 years by surveillance system and capture–recapture estimates in three US counties, 2004–2005 influenza season.
Table 1. Capture–recapture estimates of influenza hospitalization rates in children aged <5 years in three US counties, 2004–2005 influenza seasonFootnote *
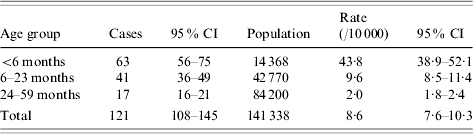
CI, Confidence interval.
* Age distribution derived from the New Vaccine Surveillance Network (NVSN) (7 d/week in-patient surveillance).
Table 2. Estimated influenza hospitalization rates and sensitivities by surveillance system in three US countiesFootnote *, 2004–2005 influenza season
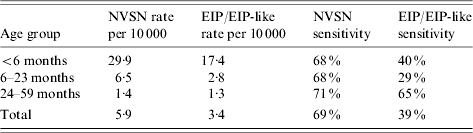
NVSN, New Vaccine Surveillance Network; EIP, Emerging Infections Program.
* Compared to capture–recapture estimates.
Influenza hospitalizations that were detected by only one of the systems were tabulated for comparison (Table 3). Nearly half of children undetected by NVSN (47%) were screened but not enrolled because they did not meet pre-defined selection criteria. Three additional children tested negative for both viral culture and RT–PCR but subsequently had positive rapid antigen detection and one was admitted to a hospital not included in the NVSN surveillance. Two children's parents did not provide consent to participate and two children were missed. For EIP/EIP-like surveillance, 68% of children that were undetected did not have an influenza diagnostic test ordered by the practitioner. An additional 30% were tested, but the tests were negative for influenza. Finally, one child was missed.
Table 3. Non-matched influenza-associated hospitalizations captured by surveillance system in three US counties, 2004–2005 influenza season
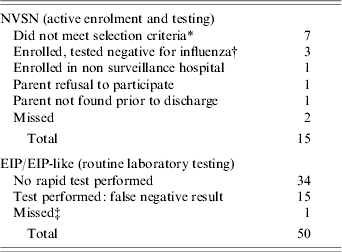
NVSN, New Vaccine Surveillance Network; EIP, Emerging Infections Program.
* Admission diagnosis: failure to thrive (FTT) [Reference Hilleman1], sickle cell crisis with fever [Reference Lavanchy2], seizures associated with Sturge Weber syndrome [Reference Hilleman1], FTT without fever [1], diabetic ketoacidosis (DKA) [1], and child abuse [Reference Hilleman1].
† Positive rapid test on tracheal aspirate in ED (prior to enrolment) [Reference Hilleman1], nasal wash [Reference Hilleman1]; and tested after 1–2 days of hospital testing [Reference Hilleman1].
‡ Same child admitted twice for two distinct episodes (one influenza A, one influenza B), the second was missed.
DISCUSSION
With continual changes in antigenic structure, as well as yearly variation in onset and severity of disease, influenza virus poses unique public health challenges. In addition, the effectiveness of influenza vaccine may vary from year to year. Thus, surveillance systems to monitor disease activity, estimate disease burden, and evaluate vaccination programmes are needed. With limited resources, alternative methodologies that combine data from ongoing surveillance systems should be considered. Using data from two independent surveillance systems that detected medically attended community-acquired influenza, we estimated the burden of influenza hospitalizations in children aged <5 years within three US counties. The application of capture–recapture methods to data from the two surveillance systems to account for those undetected cases gave a better estimate of influenza-associated hospitalizations than either system alone.
Since many patients with influenza do not have diagnostic tests performed, active enrolment and testing is necessary to avoid missing a large proportion of cases [Reference Poehling13]. NVSN performed intensive surveillance enrolling children 7 days per week and the use of both viral culture and RT–PCR provided high sensitivity for influenza detection [Reference Weinberg21]. However, despite the time and resources invested, active enrolment and testing did not accomplish complete ascertainment of influenza hospitalizations. Taking the capture–recapture calculations as the reference, 69% of the estimated total hospitalizations were correctly identified in the three participating counties. During the moderately severe 2003–2004 influenza season, a similar evaluation restricted to Davidson County, Tennessee, reported an estimated sensitivity of 73% for NVSN [Reference Grijalva11] compared to 77% during the milder 2004–2005 influenza season. The major reason for incomplete ascertainment (Table 3) was children with atypical presentations who did not meet enrolment criteria. Although respiratory symptoms were not listed as their reason for admission, it is likely that these illnesses were due to influenza (Table 3). A small number of children were missed with negative RT–PCR and viral culture at the time of hospital admission, but yielded positive results with tests that were part of their usual medical care. Additional cases were missed because of admission to a non-surveillance hospital; guardian refusal; or inadvertent exclusion.
Ascertainment of laboratory records of testing already performed as a part of routine patient care makes the EIP/EIP-like surveillance cheaper and logistically simpler than NVSN, since there is no requirement for enrolment or testing and informed consent for record review can be waived. These advantages have facilitated the implementation of EIP/EIP-like systems in several US states, encompassing a large number of participating hospitals [14, Reference Schuchat22]. Nonetheless, the detection of influenza cases by these systems, relies on the physician's decision to order an influenza diagnostic test. Children not tested will be undetected by these systems and the proportion of suspected cases tested may vary by site, season, and age group. Thus, there will be underestimation of the influenza burden, unless this type of surveillance is coupled with additional information on the proportion of true cases that are tested and the sensitivity of the diagnostic tests used.
Surveillance for cases tested as part of routine patient care detected 39% of the total number of hospitalizations, when the capture–recapture estimate was taken as reference. A similar study performed during the moderately severe 2003–2004 influenza season estimated a sensitivity of 38% for EIP in Tennessee [Reference Grijalva11] compared to 30% during the milder 2004–2005 season. The vast majority of those undetected in the EIP/EIP-like system had no influenza diagnostic test performed (Table 3). The other important factor in the lower detection rate was the lower sensitivities of commercially available rapid influenza tests (sensitivity ranges from 44% to 95%) [Reference Ruest23–Reference Cazacu26], the most frequently used test method for influenza diagnosis performed during routine patient care, compared to viral culture and RT–PCR.
For influenza surveillance systems, testing all eligible patients to obtain an accurate count of the total number of cases seeking medical care in a specific population requires a large investment of resources. On the other hand, less expensive alternatives can result in significant undercounting. New alternatives are needed to make better use of limited resources to obtain estimates with a reasonable degree of precision. A recent analysis combined administrative and laboratory data by validating subsets of putative cases through the use of gold standard tests [Reference Halloran and Longini27]. Capture–recapture analyses are another alternative that can make efficient use of more than one data source. These methods may be applied to data from two or more surveillance systems to obtain better estimates about the total number of cases. Neither of the systems needs to accomplish complete ascertainment. However, the accuracy of these estimates relies on the fulfilment of some assumptions [8].
A critical assumption for the capture–recapture analysis is that the probability of being captured by one source is not related to the probability of being detected by the other source, the independence assumption. It is possible that both sources selectively missed children who had low influenza virus shedding at the time of presentation, due to test sensitivities. The result of this scenario would be conservative, an underestimation of missed cases [8, Reference Hook and Regal17, Reference Hook, Regal, Brookmeyer and Stroup28].
Although this study encompassed three US counties, there were a relatively small number of cases identified, precluding detailed subgroup analyses. Nevertheless, our findings are consistent with previous reports and highlight the great burden of influenza in young children especially those aged <6 months. Monitoring this population is important because young children shed infectious virus for longer periods than adults, and have a crucial role in the spread of infection within the community and within the households [Reference Stephenson and Zambon4, Reference Poehling13, Reference Glezen and Couch29–Reference Rennels and Meissner31]. The year 2004 was the first in which the CDC recommended routine influenza immunization of all children aged 6–23 months. Despite vaccine shortages during the 2004–2005 season, it was estimated that 33% of US children in this age group received at least one influenza vaccine dose. However, the proportion of fully vaccinated children in this age group was 18% [32]. The burden of influenza during the 2004–2005 season is likely lower than it would have been without implementation of this new policy. In early 2006, influenza vaccination was recommended for all children aged 6–59 months and for those in contact with younger children in the United States [Reference Smith33]. Influenza surveillance systems are required to monitor the disease occurrence in this sentinel population and to assess the impact of vaccination programmes.
The two surveillance systems that operated simultaneously in three US counties would appear to give conflicting results, if reported separately. However, combining these systems gave the best estimate of disease burden, and could be a model for obtaining similar information for older children and adults. The application of capture–recapture methods to data from two or more imperfect systems provide more accurate estimates of the burden of influenza infection on hospitalizations than any single system alone. At least one of these systems would need to have a very sensitive test for influenza (such as RT–PCR), otherwise a significant proportion of cases could be missed. In addition, the current diagnostic practices of clinicians indicate that at least one system would need to actively enrol and test children, since the majority of children admitted with influenza do not currently have influenza diagnostic tests performed as part of routine care. The information provided by such a system that includes active enrolment and the use of sensitive diagnostic tests could be complemented with additional sources of information such as the less expensive clinical laboratory surveillance systems.
In 2004–2005, NVSN attempted to enrol all eligible children performing surveillance 7 days per week, but population-based estimates of rates can also be obtained using a weighted sample of eligible children [Reference Grijalva11], which reduces the resources needed. Since capture rates are not required to be similar between the participating surveillance systems, the system with the higher sensitivity (more expensive, NVSN type) could operate during a limited time (i.e. 1–4 days per week), whereas surveillance that relies on testing through routine patient care could attempt to identify all positive clinical tests. The use of capture–recapture techniques should be considered to complement the information generated by current influenza surveillance systems.
ACKNOWLEDGEMENTS
We are indebted to the collaboration of the New Vaccine Surveillance Network and the Emerging Infections Program. This research study was funded in part by the Centers for Disease Control and Prevention's New Vaccine Surveillance Network Cooperative Agreement U38/CCU417958 and Emerging Infections Program's Cooperative Agreement U50/CCU416123.
DECLARATION OF INTEREST
M.R.G. has an investigator initiated research contract with MedImmune.








