INTRODUCTION
Two main approaches to mitigate Salmonella in broiler chickens include control at slaughter and throughout processing [1] and an integrated ‘farm-to-fork’ approach with various control aspects on-farm, during catching and transport, at slaughter, and at retail and consumer levels [Reference Van Immerseel2–Reference Wegener4]. A shift in food safety philosophy has occurred over the past few decades [Reference Collins and Wall5], partly due to the success demonstrated in Denmark after the introduction of nationwide Salmonella control programmes in laying hens, broiler chickens and swine [Reference Wegener4]. In broiler chickens, this approach has been credited with a reduction in the flock-level prevalence of Salmonella from 12·9% in 1997, to 1·5% in 2002 and a concurrent decrease of 78% in Salmonella infections in people attributed to domestically produced poultry [Reference Forshell and Wierup3]. The European Union (EU) soon followed suit issuing EU regulation 646/2007 that specified targeted reductions of S. Enteritidis and S. Typhimurium in broiler flocks to ⩽1% by 31 December 2011 [6]. In Canada, Salmonella control in various food animals has traditionally followed the processing-intensive approach, primarily to maintain open borders with the USA which requires any country exporting meat and poultry products to produce under an inspection system that meets its performance criteria [1]. As such, the Food Safety Enhancement Program (FSEP) was adopted voluntarily in the 1990s and later became mandatory in December 2005 in all federally inspected processing plants [Reference Rajić7]. More recently, voluntary on-farm food-safety programmes were established in Canada in the main food-animal commodities. The Chicken Farmers of Canada follow the Safe, Safer, Safest programme which stresses good production practices (GPP) and HACCP principles but does not include a targeted Salmonella monitoring system or specific intervention strategies to control Salmonella in broilers [8]. A need for a better understanding of the effectiveness of various interventions for reducing Salmonella in broiler chicken at various stages of production has been recognized internationally [9].
Quantitative microbial risk assessment (QMRA) is an internationally endorsed framework primarily used to estimate the risk posed by microbial hazards to human health, but is also useful for evaluating the effectiveness of intervention strategies, as well as identifying critical knowledge gaps in systems of interest [Reference Toyofuku10]. The overall utility of QMRA depends on the inputs used to inform the framework, which should be valid, precise and transparent [Reference Goldbohm11–Reference Peters13]. The synthesis research framework, and methods such as scoping studies (ScS), systematic review (SR) and meta-analysis (MA) offer structured and repeatable methods for collecting, mapping, including/excluding, appraising and synthesizing evidence related to a topic of interest [Reference Anderson14–Reference Sargeant16] and can complement and enhance the robustness of QMRA.
The study presented here was part of a larger initiative aimed at developing an evidence-based framework, linking specific synthesis research and QMRA methods, for evaluation of potential control options against Salmonella in broiler chickens from farm-to-processing within the Ontario, Canada context. The main objective of this study was to develop a quantitative exposure assessment (QEA – a component of the QMRA process) capable of evaluating the effectiveness of intervention strategies for reducing Salmonella concentration [(colony-forming units (c.f.u.)/carcass] and prevalence in broiler chickens from the level of the grow-out farm to the end of primary processing (chilling). A secondary objective was to briefly demonstrate the use of research synthesis methods to generate inputs for QEA, and comment on their suitability for informing various aspects of QEA.
METHODS
Model description
The model consisted of three modules: (1) the grow-out farm, (2) transport (3) and primary processing (Fig. 1), beginning at the grow-out farm with a flock of broiler chickens externally contaminated with Salmonella and ending with an estimation of the mean c.f.u./carcass and prevalence of Salmonella on a group of 100 carcasses after chilling. The framework for the model was adapted from a previously published QMRA that used a mechanistic modelling approach in its exposure assessment to follow changes in number of Campylobacter spp. from farm to the consumer [17]. This approach provided a straightforward means of understanding the different relationships between steps in the production system and the model was readily modifiable to our question.
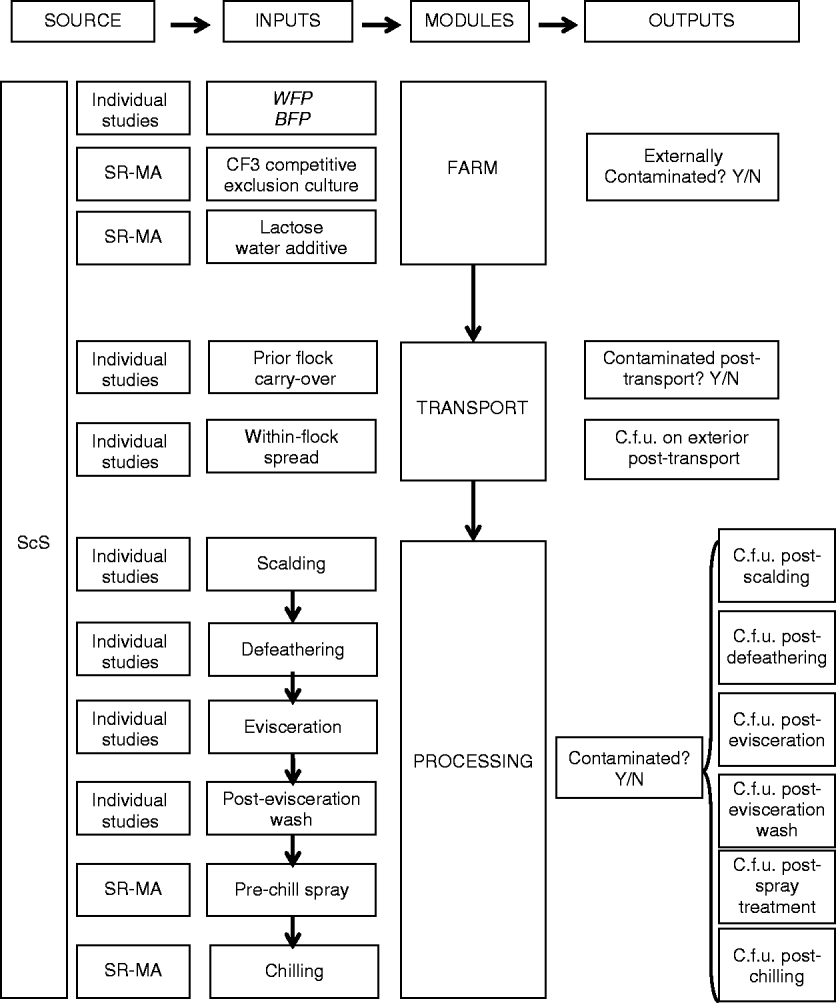
Fig. 1. Sources of information, and inputs used to inform the quantitative exposure assessment model framework describing the grow-out farm-to-secondary processing pathway of the Ontarian broiler chicken industry. ScS, Scoping study; SR-MA, systematic review-meta-analysis; WFP, within-flock prevalence; BFP, between-flock prevalence; CF3, continuous flow culture 3, c.f.u., colony-forming unit.
The model was developed using @Risk 5.0 (Palisade, USA), a Microsoft Excel add-on. Monte Carlo sampling procedures were used for 10 000 iterations per model simulation with the same initial seed chosen randomly by the software.
Model inputs
As much as possible, data used to inform intervention and prevalence aspects of the model were obtained from complementary ScS and SR-MA (Fig. 1). The methods and respective results are reported in detail elsewhere and can be made available upon request from the corresponding author [Reference Farrar18, Reference McEwen19]. Briefly, the ScS was used to identify and organize the global scientific evidence for interventions and prevalence estimates of Salmonella in broiler chickens as well as to select interventions for further evaluation through SR-MA. A research team evaluated a resulting list of interventions (Fig. 2) and based on the overall amount of primary research available, basic study design characteristics and contextual suitability of conducted studies, selected eight relatively broad intervention categories for concurrent and independent investigation through SR-MA. These were competitive exclusion (CE), other feed and water additives (except for CE and antimicrobials), vaccination, biosecurity, carcass scalding, post-evisceration washing, pre-chill spraying/dipping and chilling [Reference Farrar18, Reference McEwen19]. Other interventions were not considered for one or more reasons. For example, antimicrobials were not considered due to animal and public health concerns associated with antimicrobial resistance [Reference Viola and DeVincent20–Reference Silbergeld, Graham and Price22]. An insufficient amount of research existed for some potentially promising interventions (e.g. bacteriophage, reprocessing) and large heterogeneity was frequently observed precluding the use of MA [Reference McEwen19]. One exception was the biosecurity category selected for SR-MA because these practices are routinely recommended to producers [Reference Van Immerseel2, Reference Fraser23], even though few studies with a large amount of heterogeneity were indentified for this category. Three criteria used to select interventions for complementary QEA were:
(1) MA could be performed on a specific intervention treatment (⩾2 studies reporting the effectiveness of the same intervention treatment, in similar chicken populations, measuring the same outcome, repeatable under commercial conditions, that could be given as a recommendation to producers or processors) resulting in significant Salmonella reductions.
(2) When MAs were possible for multiple treatments within the same intervention category, the treatment supported by more studies, the larger effectiveness and a continuous outcome was selected. For example, within the pre-chill carcass spray/dip category, a trisodium phosphate (TSP) spray was chosen for inclusion in the QEA because it had a continuous outcome, the largest reductions in Salmonella and the second highest number of studies underpinning its effects.
(3) Evidence derived from controlled trial studies carried more weight than from challenge trial studies.
A final list of the interventions selected for incorporation into the QEA and the main characteristics of their respective data are shown in Table 1. Significant heterogeneity and/or publication bias was consistently observed for most interventions [Reference McEwen19] so we decided to report information for these aspects instead of using them as exclusion criteria. Due to a lack of concentration (e.g. c.f.u./unit) data at the farm level, MA estimates based on prevalence data were used to populate farm intervention inputs, while MA estimates based on concentration data were used to inform processing intervention inputs. A significant lack of intervention studies reporting concentration outcomes, conducted under commercial (‘real-life’) conditions was noted during the conduct of the complementary SR-MAs [Reference McEwen19], so concentration data from pilot plant or laboratory studies were chosen and acknowledged to carry some uncertainty with regard to their representation of true effects under field conditions. When the impact of an intervention was measured against Salmonella prevalence, the pooled MA effect estimate used was the risk difference (RD), converted from a pooled OR (odds ratio) [Reference McEwen19]:
where RD=the risk difference (i.e. number of carcasses per 100 carcasses processed that are unlikely to become Salmonella contaminated in the treatment group compared to the untreated group); OR=odds ratio; and ACR=assumed control/baseline risk.
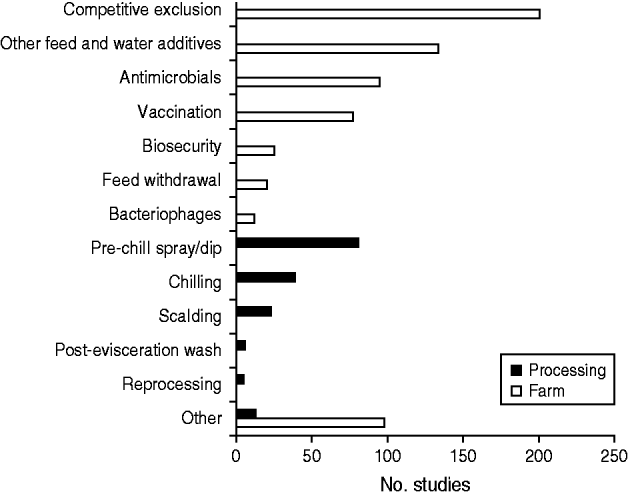
Fig. 2. Various interventions identified through our scoping study with the amount of primary research underpinning each.
Table 1. Selected interventions [based on systematic review-meta analysis (MA)] and their respective inputs for the quantitative exposure assessment model
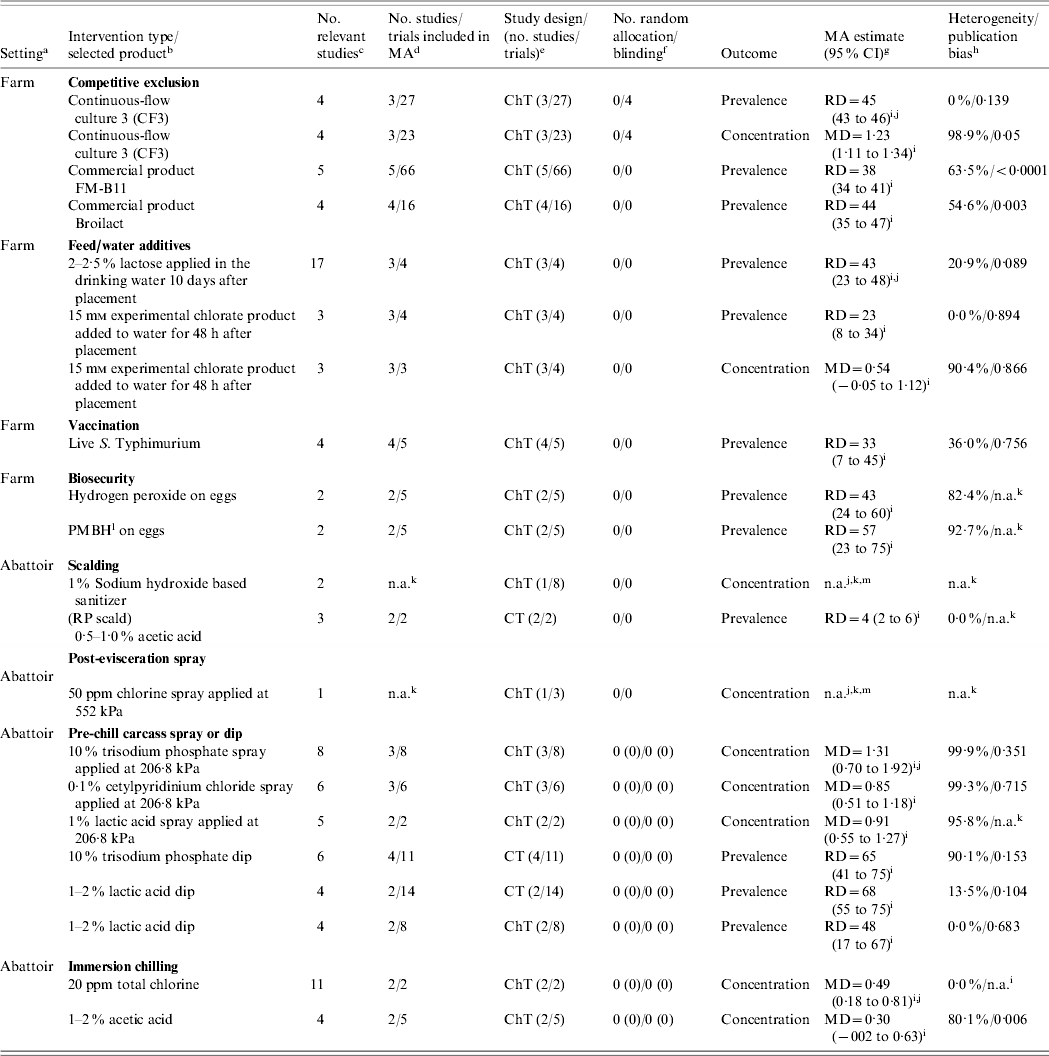
a Intervention application point.
b For each intervention type up to three datasets were selected to represent intervention profile. The selection was based on a combination of arbitrary, biological and contextual criteria.
c Studies (papers) confirmed relevant during the SR-MA process.
d Number of relevant studies (trials) for each intervention type or product that were included in random-effect MA.
e ChT, Challenge trial; CT, controlled trial.
f Random allocation of intervention/concealment of treatment. Randomized and/or non-randomized clinical or field trials and/or challenge trials.
g RD, Risk difference; MD, mean difference.
h Statistical significance of heterogeneity (⩾25%) and publication bias (⩽0·1) as measured through Egger's regression asymmetry test.
i Calculated from MA.
j Selected for inclusion in the quantitative exposure assessment.
k Not applicable.
l PMBH, Polyhexamethylenebiguanide hydrochloride.
m Calculated from individual studies.
To convert ORs to RDs an ACR of 21·2% [Reference Arsenault24] was employed since it provided the most up-to-date estimate of Salmonella prevalence at processing, in Canada, at the time. Typically, a range of ACRs are used to explore the impact of alternative baseline risks on the results [25]; however, the outputs from our MAs are intended for use as inputs for our QEA, so a single ACR was used instead. When the impact of an intervention was measured against Salmonella concentration, the pooled MA effect estimate used was the raw mean difference (MD) [Reference McEwen19].
When data were unavailable from complementary SR-MAs, individual studies captured through our ScS, or at the authors' discretion were used to populate prevalence and concentration data inputs. Priority was given to prevalence estimates obtained from within Canada and published after 2000. If necessary, this criterion was relaxed to include data from the USA.
When populating intervention aspects of the QEA, probability distributions were constructed from pooled estimates of effectiveness and their respective 95% confidence intervals (CIs) when heterogeneity was not significant. When heterogeneity was significant outputs generated from MA (reported outcome, its respective 95% CI and associated weight) were used to capture the variability and uncertainty in the data and construct probability distributions for use in the QEA. An alternative to this may also be to use pooled estimates of effectiveness and their respective 95% prediction intervals as the prediction interval will also capture variability in the data [Reference Borenstein26].
Intervention scenarios
In order to model the impact of interventions employed on-farm and at processing, 12 model scenarios were constructed; six scenarios for carcasses destined for the fresh product market and six scenarios for carcasses destined for the frozen product market:
(1) A baseline scenario without interventions on farm or during processing (scalding, post-evisceration spraying, pre-chill spraying and immersion chilling employed potable water).
(2) Incorporating the effect of a competitive exclusion culture [continuous flow culture 3 (CF3)] on-farm to reduce within-flock prevalence (WFP) by the end of the grow-out module.
(3) Incorporating the effect of lactose as a water additive on-farm to reduce WFP by the end of the grow-out module.
(4) Incorporating all processing-level interventions to reduce the concentration of Salmonella on carcasses at scalding, post-evisceration washing, pre-chill spraying and chilling.
(5) Incorporating both farm (CF3 only) and processing interventions to reduce the WFP on-farm as well as concentration of Salmonella at processing.
(6) Incorporating the effect of a competitive exclusion culture CF3 to reduce WFP by the end of the grow-out module as well as hypothetical reductions in between-flock prevalence (BFP), also known as flock-level prevalence, and c.f.u./broiler of 50% by the end of the grow-out and post-transport modules, respectively.
Scenario results were displayed as c.f.u./carcass and prevalence of Salmonella at the end of chilling. The relative reduction (RR) in Salmonella concentration and prevalence in comparison to baseline results was calculated for each scenario. Within each scenario, estimates of concentration and prevalence were generated for populations of broilers originating from both positive and negative flocks.
Model assumptions
A series of assumptions were made to reduce as much complexity/uncertainty in the model as possible:
• Potential changes in Salmonella contamination or production practices and their effects are equally probable in birds belonging to the same flock.
• There is uniform mixing in the population of broiler chickens within the same flock (i.e. Salmonella contamination or the effect of an intervention is not localized to clusters of birds within the flock).
• Birds are not lost to mortality or condemnation during grow-out, transport or processing.
• On-farm production practices are similar between Canada and the USA, such that estimates of WFP are equivalent.
• Regardless of whether a bird was contaminated before or during transport, the number of Salmonella c.f.u. contaminating a bird's exterior remains unchanged.
• A steady-state of contamination/reduction of Salmonella during processing occurs by the time the 100th bird is processed.
• Direct methods of quantification of Salmonella are comparable to counts determined by most probable number (MPN) [Reference Kotula and Pandya27], so quantitative data reporting MPN are assumed to be equivalent to c.f.u.
• A positive flock is a flock containing one or more birds contaminated with Salmonella at the end of the grow-out period. A negative flock is a flock with no Salmonella-contaminated birds at the end of the grow-out period.
Exposure assessment: farm
The aim of the farm module was to estimate BFP and WFP of Salmonella for a random, Ontarian poultry flock by the time the live birds are caught for transport. Contamination was modelled at both the flock and individual bird level based on exterior contamination with Salmonella via faecal material. It was assumed that once a bird becomes contaminated with Salmonella it remains contaminated. Data from Canada and the USA were used to estimate BFP and WFP, respectively [Reference Arsenault28–Reference Siemon, Bahnson and Gebreyes32]. Beta distributions, which are typically used to describe our uncertainty about prevalence estimates [Reference Vose33], were used to capture uncertainty in the data and stochastically estimate values for BFP and WFP (Table 2).
Table 2. Description of the variables used to model Salmonella status in broiler chickens at the grow-out farm
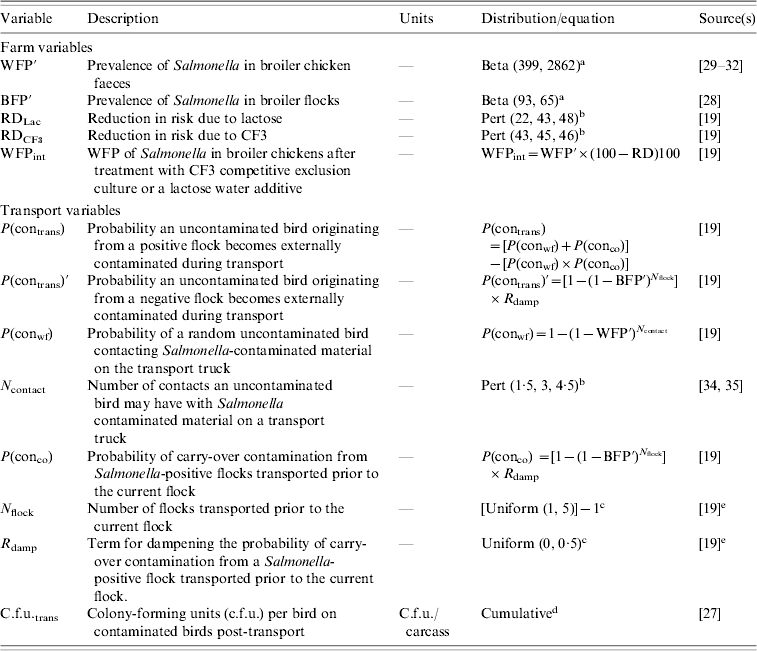
a Beta (α, β).
b Pert (minimum, most likely, maximum).
c Uniform (minimum, maximum).
d Cumulative (minimum, maximum, range of values, cumulative probability of each value in range).
e Values chosen based on authors' discretion.
To capture the impact of selected on-farm interventions, pooled estimates of effectiveness calculated in our complementary SR-MAs [Reference McEwen19] were used to inform the model of their impact on the estimated WFP (Table 2).
Exposure assessment: transport
At transport, the unit of interest switched to the individual bird with changes in Salmonella-positive/-negative status and number of c.f.u./carcass on the 100th bird belonging to a positive or negative flock followed separately for the remainder of the modelling pathway. Here the model considered the spread of Salmonella between contaminated and uncontaminated birds within a positive flock, as well as the probability of carry-over contamination from transport crates contaminated by Salmonella-positive flocks transported prior to the current flock. Birds from negative flocks are, by definition, uncontaminated by Salmonella so it was assumed that the only possibility for these birds to become contaminated during transport was from carry-over contamination of a previously transported flock. Assignment of Salmonella-positive/-negative flock and individual bird status was based on the WFP at the end of the farm module while the BFP estimated at the end of the farm module fed into the probability of a previous flock transported prior to the current flock being positive/negative (Table 2). Once a bird had been designated Salmonella positive, the concentration of Salmonella on that bird's exterior was estimated by fitting an empirical Cumulative distribution to the concentration data (Table 2) based on data from another study [Reference Kotula and Pandya27]. Since 100% of the Salmonella contamination from a prior flock will not contaminate the subsequent flock being transported [Reference Rigby34, Reference Rigby35] a term to dampen the probability of contamination was incorporated into the probability of carry-over contamination from Salmonella-positive flocks transported prior to the current flock and stochastically estimated using a Uniform distribution (Table 2).
Exposure assessment: processing
The processing module began at the scalding process and included defeathering, evisceration, post-evisceration washing, pre-chill spraying and chilling. In addition to the division between positive and negative flocks, populations of birds destined for the fresh or frozen product markets were split and followed separately based on the scalding and chilling treatments received. Birds destined for the fresh product market were soft-scalded (50°C for 90 s) and air chilled while birds destined for the frozen product market were hard-scalded (56°C for 45 s) and immersion chilled. Changes in the positive/negative status of a carcass were followed throughout the processing module by means of a conditional statement based on the estimated changes in Salmonella c.f.u./carcass after each processing step.
A lack of quantitative effect estimates from SR-MA for carcass scalding prevented a measure for the magnitude of effect of soft scalding on Salmonella c.f.u./carcass. Instead, the magnitude of effect on a surrogate organism, Campylobacter spp., was adopted for Salmonella (Table 3) to provide a starting point for which the difference between soft scalding and other scalding interventions could be calculated. The use of surrogate data at this step in the process means that it is not advisable to judge or make conclusions about the absolute effect of scalding as an intervention against Salmonella using the results from this model. However, measuring the relative effect of the other interventions should still be possible because scalding is equally applied in all the scenarios. Data describing changes in Salmonella c.f.u./carcass during carcass defeathering were also unavailable, instead, data examining theses effects on a marker organism, Escherichia coli K12, were used [17]. In this case, the marker organism was used to mechanistically describe the spread of contamination in the equipment. As such, as long as the spread of contamination in the machine is primarily a result of the physical action of the machine and not so much the nature of the organism (for instance growth or development of biofilms in the equipment) then the use of the marker organism data is not likely to be highly problematic.
Table 3. Description of the variables used to model Salmonella status in broiler chickens during processing
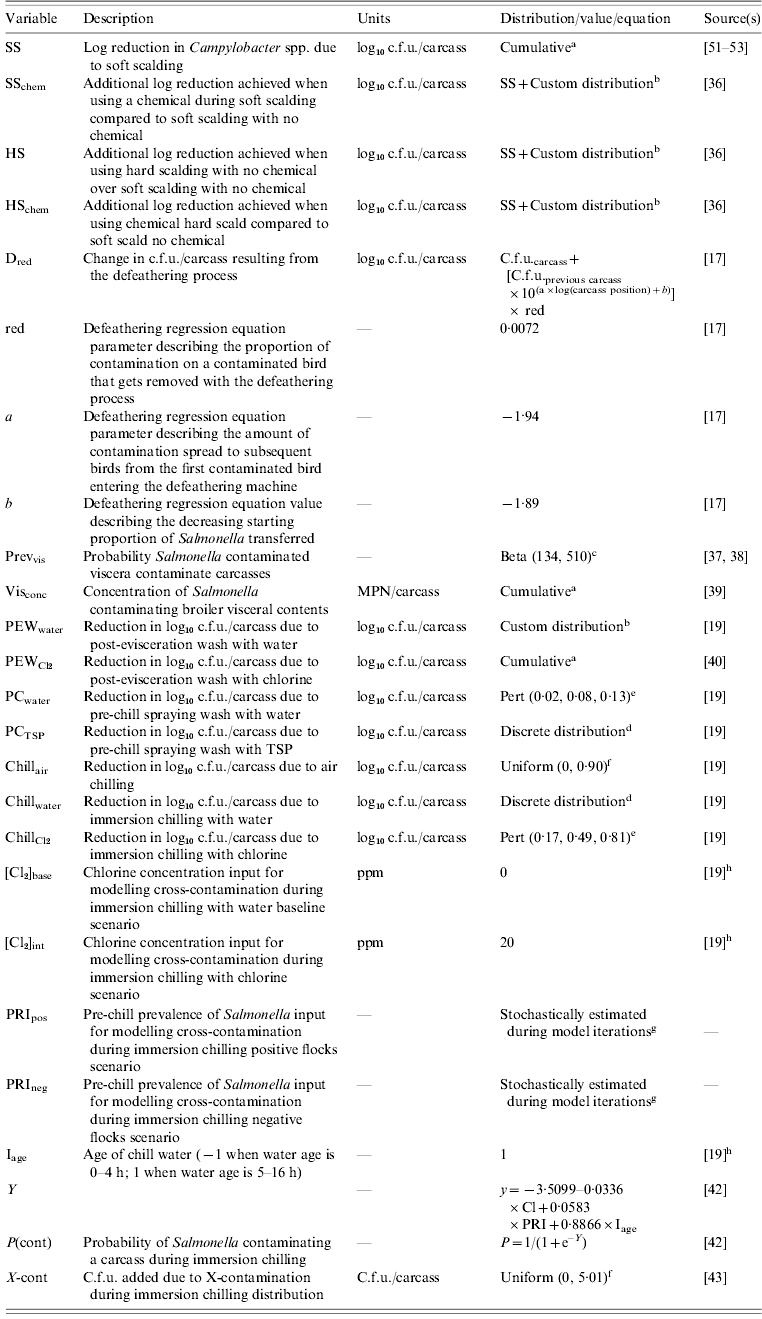
a Cumulative (minimum, maximum, range of values, cumulative probability of each value in range).
b Custom distribution consisting of outputs from a Cumulative and Pert distributions informing inputs of a Discrete distribution.
c Beta (α, β).
d Discrete (range of values, probability of each value's occurrence).
e Pert (minimum, most likely, maximum).
f Uniform (minimum, maximum).
g Each iteration of the model produces a different outcome.
h Values chosen based on authors' discretion.
Only one study [Reference McKee, Townsend and Bilgili36] reported effects of hard and soft scalding on concentration precluding MAs for these interventions, therefore data from the single study were used to calculate proportional reductions in Salmonella c.f.u./carcasses instead of pooled estimates of effect (Table 3). Differences in c.f.u. reductions due to hard and soft scalding were calculated in relation to a baseline reduction in Campylobacter spp. c.f.u. To stochastically model in the proportional reduction of Salmonella c.f.u./carcass, custom-made uncertainty distributions derived from a combination of Cumulative, Pert and Discrete distributions were utilized as model inputs to capture both the uncertainty and variability in the data [Reference Vose33] (Table 3).
The defeathering step mechanistically described cross-contamination during the defeathering process by accounting for (1) the proportion of Salmonella removed from a carcass's exterior as a result of removing contaminated feathers, (2) the potential transfer of Salmonella from a contaminated carcass to subsequent carcasses and (3) the decline in the amount of Salmonella transferred with increasing distance between a given carcass and the nearest contaminated carcass down the line. A regression model describing the amount of contamination a carcass receives as a function of the distance between it and the initial contaminated carcass was constructed [17] and used to inform the current model (Table 3).
The evisceration step considered the possibility of cross-contamination from the interior of Salmonella-colonized carcasses onto the exterior by modelling the change in Salmonella c.f.u. as a result of damage that may have occurred during the evisceration process. Data investigating the occurrence of visceral damage and Salmonella in broiler viscera were obtained through our complementary ScS search [Reference Powell37, Reference Beers38] (Table 3) and modelled using a Beta distribution. Damage to viscera in a Salmonella-colonized bird was assumed to increase Salmonella c.f.u./carcass. The amount of Salmonella in 1 g of visceral contents contaminating a carcass was stochastically estimated using a Cumulative distribution derived from the results of a single study [Reference Cason39] reporting the log10 MPN/g Salmonella in visceral contents (Table 3).
Complementary SR-MA provided estimates of log10 c.f.u. reductions in Salmonella on broiler carcasses when given a post-evisceration wash with potable water [Reference McEwen19], while an individual study provided data for a post-evisceration wash with chlorine [Reference Northcutt40]. Due to significant heterogeneity, the use of a pooled MA effect estimate to relate the effectiveness of water spraying was precluded; instead estimates reported in individual studies were fitted to Pert distributions to describe our uncertainty about the fixed values [Reference Vose33] and weighted according to those assigned to individual studies in the MA. Together with the weights, the list of potential log10 c.f.u./carcass reductions was used to capture our uncertainty about the frequency of the fixed values with a Discrete distribution [Reference Vose33] (Table 3). The single study [Reference Northcutt40] investigating a post-evisceration wash with chlorine, reporting a concentration outcome, was used to calculate proportional reductions in Salmonella c.f.u./carcass instead of pooled estimates of effect (Table 3).
Complementary MA results indicated that the subset of data investigating the log10 c.f.u./carcass reductions of a pre-chill spray with potable water did not have significant heterogeneity so a pooled MA estimate was used to derive a Pert distribution for use in the model (Table 3). An alternative pre-chill spray with TSP was selected for investigation in the model and incorporated using the same Pert and Discrete distribution combination as indicated above for the post-evisceration wash with potable water (Table 3).
The chilling module of the model considered the reduction in Salmonella c.f.u./carcass on broiler carcasses, and the possibility of cross-contamination if immersion chilling was used instead of air chilling. A single study [Reference Huezo41] investigating the change in concentration due to air chilling was identified through our complementary SR-MAs and a Uniform distribution, which is used when very little data are available [Reference Vose33], used to describe the uncertainty in this data (Table 3). MA relating the effectiveness of immersion chilling in water on the concentration of Salmonella on broiler carcasses was precluded due to significant heterogeneity. Again, a Discrete distribution, populated by outputs from a Pert distribution as mentioned previously, was used to model the change in c.f.u./carcass as a result of immersion chilling with potable water (Table 3). Conversely, heterogeneity was not significant for MA conducted on immersion chilling treatments with chlorine so a Pert distribution was used to model the change in c.f.u./carcass as a result of immersion chilling with chlorine (Table 3). The potential for cross-contamination during immersion chilling operations was investigated previously [Reference Yang42] and a model predicting the occurrence of cross-contamination based on chlorine concentration, chill water age and the pre-chill prevalence of Salmonella was derived and used in the current model to derive the probability of cross-contamination from immersion chilling (Table 3). A single study retrieved by our complementary SR-MA indicated an increase of 0·7 log10 c.f.u./carcass could be acquired by a single carcass as a result of cross-contamination [Reference Smith, Cason and Berrang43]. Our uncertainty in this estimate was modelled using a Uniform distribution (Table 3).
Sensitivity analysis
A sensitivity analysis was conducted to determine the relative impact of various model parameters on the concentration and prevalence of Salmonella at the end of chilling by ranking Spearman correlation coefficients between each of the model inputs and the final model outputs.
RESULTS
Farm interventions (CE culture CF3 and lactose water additive) scenario
Tables 4–7 summarize the effects of the different farm intervention strategies on the mean c.f.u./carcass and prevalence of Salmonella from birds originating from positive or negative flocks, destined for either fresh or frozen product markets. Farm-level interventions were only useful for birds originating from positive flocks and had a limited impact on c.f.u./carcass and prevalence of Salmonella by the end of chilling. Relative reductions in Salmonella prevalence ranged from 6·01% to 8·28% while RRs in c.f.u./carcass ranged from 13·29% to 26·86% (Tables 4 and 6). Very little difference in effectiveness was observed between the lactose and CF3 farm interventions with average reductions in WFP of 41% and 45%, respectively.
Table 4. Estimated mean c.f.u./carcass and prevalence of Salmonella in broiler carcasses after chilling originating from a positive flock, destined for the fresh product market after implementing selected interventions along the broiler production chain
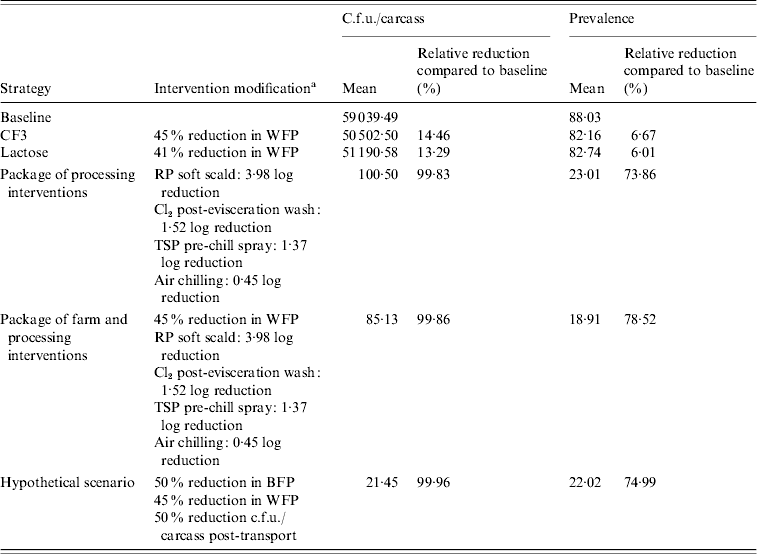
a WFP, Within-flock prevalence; RP scalding, soft scalding with a 1% sodium hydroxide-based sanitizer; TSP, trisodium phosphate.
Processing interventions scenario
Processing-level interventions had much larger impacts than farm-level interventions on reducing Salmonella c.f.u./carcass and prevalence. Birds destined for the fresh product market showed up to 99·83% RRs in Salmonella c.f.u./carcass while RRs in prevalence ranged from 73·86% to 87·78% (Tables 4 and 5). A similar trend in reductions was observed for birds destined for the frozen product market with extremely high RRs in c.f.u./carcass of 99·84% and 89·94% for positive and negative flocks, respectively and RRs in prevalence of 44·46–43·88% in positive and negative flocks, respectively (Tables 6 and 7).
Table 5. Estimated mean c.f.u./carcass and prevalence of Salmonella in broiler carcasses after chilling originating from a negative flock, destined for the fresh product market after implementing selected interventions along the broiler production chain
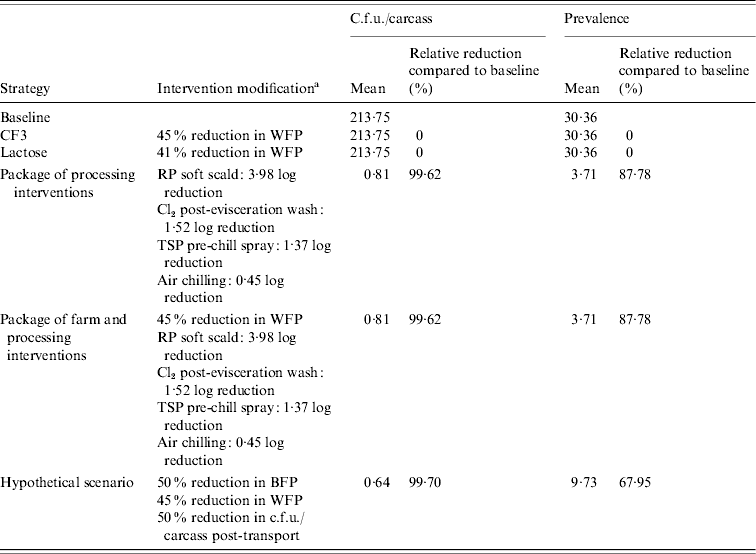
a WFP, Within-flock prevalence; RP scalding, soft scalding with a 1% sodium hydroxide-based sanitizer; TSP, trisodium phosphate; BFP, between-flock prevalence.
Table 6. Estimated mean c.f.u./carcass and prevalence of Salmonella in broiler carcasses after chilling originating from a positive flock, destined for the frozen product market after implementing selected interventions along the broiler production chain
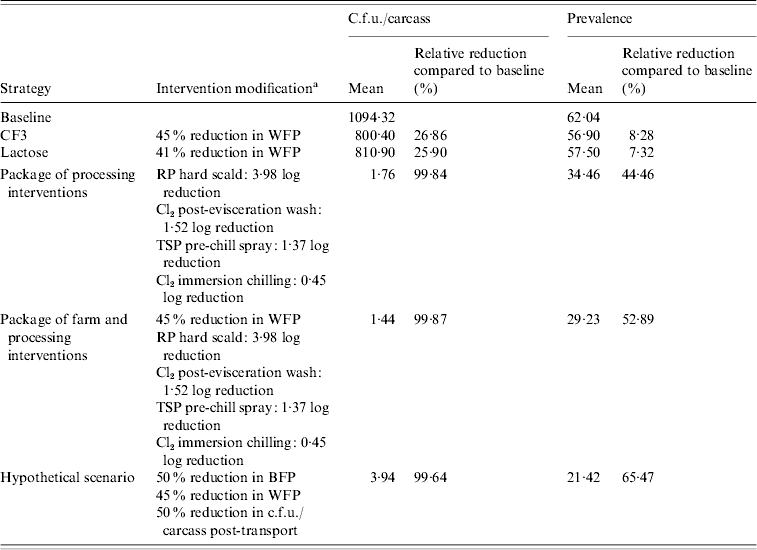
a WFP, Within-flock prevalence; RP scalding, soft scalding with a 1% sodium hydroxide-based sanitizer; TSP, trisodium phosphate; BFP, between-flock prevalence.
Farm and processing interventions scenario
Slightly higher RRs in Salmonella c.f.u./carcass and prevalence for the package of farm and processing interventions, compared to processing interventions alone, were estimated. Relative reductions in c.f.u./carcass ranged from 99·86% to 99·87% while RRs in prevalence ranged from 52·89% to 78·52% in birds originating from positive flocks (Tables 4 and 6). Birds originating from negative flocks did not receive any added benefit from a package of farm and processing interventions compared to processing interventions alone (Tables 5 and 7).
Table 7. Estimated mean c.f.u./carcass and prevalence of Salmonella in broiler carcasses after chilling originating from a negative flock, destined for the frozen product market after implementing selected interventions along the broiler production chain
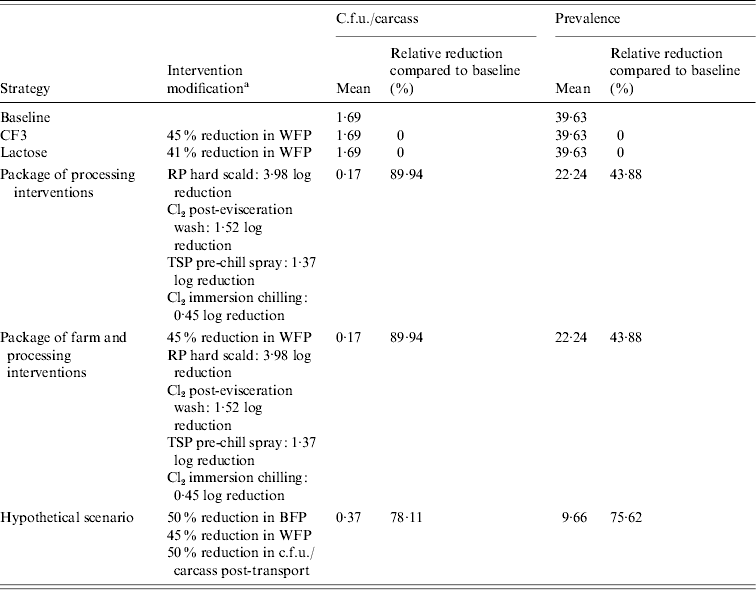
a WFP, Within-flock prevalence; RP scalding, soft scalding with a 1% sodium hydroxide-based sanitizer; TSP, trisodium phosphate; BFP, between-flock prevalence.
Hypothetical farm and transport interventions scenario
Very large RRs in Salmonella c.f.u./carcass and prevalence were observed when we incorporated reductions in BFP and c.f.u./carcass post-transport in combination with reductions in WFP from the CF3 intervention. Slightly higher RRs in c.f.u./carcass (99·96% and 99·70%) were observed for populations of birds destined for the fresh product market originating from positive and negative flocks in this scenario compared to those in the processing intervention and farm and processing intervention scenarios (Tables 4 and 5), although RRs in prevalence were not as impressive (74·99% and 67·95%) for positive and negative flocks, respectively. The opposite was observed in populations of birds destined for the frozen product market. Relative reductions in c.f.u./carcass (99·64% and 78·11%) were lower, for positive and negative flocks respectively, in this scenario compared to those in the processing intervention and farm and processing intervention scenarios (Tables 6 and 7), although RRs in prevalence were larger (65·47% and 75·62%) for positive and negative flocks, respectively.
Sensitivity analysis
Figures 3 and 4 present the results of a sensitivity analysis in the baseline population of broilers destined for fresh and frozen product markets originating from positive or negative flocks. For fresh product markets from a positive or negative flock, the most influential parameters on the c.f.u./carcass of Salmonella at the end of chilling were the scalding reduction, whether birds entered the defeathering machine contaminated with Salmonella and the reduction due to the post-evisceration wash (Fig. 3). For birds destined for the frozen product market, in addition to the reduction due to scalding, contamination changes in c.f.u./carcass during chilling also had a major influence on the final c.f.u./carcass of Salmonella at the end of chilling.

Fig. 3. Tornado charts of broilers destined for fresh [panels (a) and (b)] and frozen [panels (c) and (d)] product markets originating from positive (a, c) and negative (b, d) flocks with Salmonella concentration (c.f.u./carcass) at the end of chilling as the output of interest.
Similar influential parameters on the prevalence of Salmonella at the end of chilling were observed for broilers destined for fresh products originating from positive or negative flocks (Fig. 4) with the reductions due to scalding, contamination entering the defeathering machine and a post-evisceration wash again having a large influence. For birds destined for the frozen product market, the impact of chilling was very influential for both positive and negative flocks (Fig. 4).

Fig. 4. Tornado charts of broilers destined for fresh [panels (a) and (b)] and frozen [panels (c) and (d)] product markets originating from positive (a, c) and negative (b, d) flocks with Salmonella prevalence at the end of chilling as the output of interest.
DISCUSSION
As expected, the combination of on-farm and processing interventions was more effective than single interventions with respect to RRs in both Salmonella c.f.u./carcass and prevalence by the end of primary processing. Although this scenario did not incorporate our hypothetical reductions in BFP and c.f.u./carcass post-transport, substantial reductions in Salmonella by the end of primary processing were still achieved.
The addition of on-farm interventions to reduce the WFP of Salmonella prior to transport resulted in a limited reduction in Salmonella c.f.u./carcass and prevalence on broiler carcasses by the end of chilling; however, these results were derived using the only data available that reported effects at the within-flock level and did not test the effect of reductions in c.f.u./broiler pre-transport. When we incorporated hypothetical interventions on farm that could reduce BFP and c.f.u./broiler post-transport by 50% each, RRs in Salmonella c.f.u./carcass and prevalence were comparable or higher, in some populations of broilers, than with processing interventions alone, or both farm and processing interventions, thus highlighting the potential importance of Salmonella control prior to processing. A lack of data reporting naturally occurring concentrations of Salmonella in broiler chickens on-farm and prior to transport precluded use of the model to estimate changes in c.f.u./broiler prior to transport and may have resulted in the limited effectiveness of farm interventions. Since the model could not estimate c.f.u./broiler pre-transport, any bird estimated to be positive after the transport module was assigned a level of contamination as previously reported [Reference Kotula and Pandya27]. Because the c.f.u./carcass of contamination (concentration) reported by Kotula & Pandya [Reference Kotula and Pandya27] were so high, the reductions in WFP estimated on-farm had little impact on the final c.f.u./carcass and prevalence of Salmonella after chilling. As seen by comparing the final c.f.u./carcass and prevalence of Salmonella after chilling between birds originating from positive and negative flocks, or between scenarios where WFP and WFP, BFP and c.f.u./carcass post-transport were reduced, substantial reductions of about 2 log10 c.f.u./carcass and 22–58% prevalence can potentially be achieved with sufficient reductions of Salmonella on-farm. Data limitations restricted the model from fully evaluating the effects of implementation of farm-level interventions on Salmonella c.f.u./carcass and prevalence by the end of primary processing. Future research should focus on determining naturally occurring concentrations of Salmonella in broiler chickens on-farm and on determining the effects of on-farm interventions at the flock level so more informed modelling can be completed in the future.
In our processing intervention only scenario, interventions implemented as a package resulted in larger reductions of Salmonella c.f.u./carcass and prevalence by the end of chilling compared to our baseline scenario. This may be due to the effects of processing interventions being directly linked and additive. However, it should be noted that the observed reductions may be exaggerated given that data used to populate the model were not from field trials and the magnitude of effect of the scalding and defeathering operations were informed using surrogate data.
Sensitivity analyses are highly dependent on the range of values used as model inputs so results should be interpreted within the context of the model. Our analysis indicated that the scalding reduction, contamination during defeathering, the reductions during post-evisceration washing and the potential for cross-contamination during chilling were highly influential on the final c.f.u./carcass and prevalence of Salmonella by the end of chilling. It is important to note that the scalding reductions and degree of cross-contamination during defeathering were based on surrogate data from Campylobacter and Escherichia coli, so these parameters do not necessarily represent the absolute effect of scalding or defeathering on Salmonella. A more reasonable representation of these parameters might be the level of contamination at the farm or any other intervention that reduces Salmonella contamination prior to entry into the processing plant. As evidenced by our hypothetical scenario, any intervention prior to processing that achieved the reductions estimated for scalding would be almost as effective provided other things did not happen after that step to negate their effect, for example, the equivalent log reduction at the farm would be just as influential as scalding, provided there was no subsequent increase during transport.
Due to a lack of data, a number of assumptions were made to complete the model, therefore it should be emphasized that the utility of the model does not lie in its ability to fully characterize the industry situation, but rather in improving our understanding of the different interactions between different steps of production within the Canadian broiler production system in general, and to estimate the effect of promising interventions regardless of their current use in industry. For example, the process risk modelling approach [Reference Cassin44, Reference Fazil45] to create exposure assessments for risk modelling relies on the outputs generated from one module to inform the inputs of subsequent modules which enables a deeper understanding of the interactions among the process/modules in a system. Using the model, we compared scenarios based on the implementation of different intervention strategies with a baseline scenario. Using this approach, any added uncertainty due to assumptions about intervention effectiveness (i.e. the use of surrogate organisms for the scalding and defeathering processes) would be applied equally across all scenarios and therefore should not detract from the model's utility for evaluating different intervention strategies. Interpretations were therefore made on a relative basis and in consideration of the model assumptions instead of on an absolute basis.
Another consideration is that the interventions chosen for incorporation into this QEA are based on the available primary research and our ability to synthesize it using our SR-MA methods. During the conduct of our complementary SR-MAs a lack of commercially conducted studies was noted [Reference Farrar18, Reference McEwen19] so studies investigating experimental interventions conducted under laboratory or pilot plant conditions with promising results were chosen for incorporation into the QEA instead.
We observed some challenges regarding the application of ScS and SR-MA within the context of QMRA. The main one concerned the consistently observed problems encountered in methodological soundness and/or reporting completeness of primary research articles used for generating various types of data needed for formal synthesis. The large number of unique combinations of study design-outcome measurement-intervention treatments often precluded more robust MA, contributed to significant heterogeneity when MA was appropriate, or to significant publication bias limiting the reliability of a pooled estimate of effectiveness. An additional challenge was the considerable manpower, expertise and time demands of this integrated synthesis research-risk assessment approach. The time required to undertake the process depends greatly on the complexity of the issue (question), availability of experienced manpower and expertise (e.g. library resources) and the extent of involvement from stakeholders in the implementation process.
Overall, we found that synthesis research methods such as ScS and SR-MA clearly highlighted the sources and lack of evidence available to inform our QEA and could provide end-users with a better understanding of the decision-making process for data inclusion/exclusion and offer a broader context from which conclusions and recommendations could be made. As such, we believe these methods should be considered for inclusion within the existing QMRA frameworks of agencies at national and international levels. The European Food Safety Authority (EFSA) has already adopted a Guide for SRs that will be used for their risk assessments [46]. Scoping studies are similar to narrative reviews in that they both collect, synthesize and interpret existing literature to address broad, complex issues but differ in that they focus on describing the breadth and nature of an existing body of evidence, rather than address or answer a specific issue or question. In addition, they include a wide range of research and non-research materials, such as qualitative and quantitative peer-reviewed research as well as formal and informal commentaries [Reference Davis, Drey and Gould47]. As such, ScS rarely include a methodological assessment of the included literature so are better suited as preliminary investigations for developing more focused research questions that can be addressed using more in-depth research methods. Even though a clear consensus on the ScS framework has yet to be reached, we believe that this format was very useful for mapping the key aspects underpinning the broad, complex research area of Salmonella contamination of broilers, and could be more widely applicable to other complex food safety problems [Reference Arskey and O'Malley48, Reference Levac, Colquhoun and O'Brien49]. Others have described ScS as a ‘semi-quantitative methodology for objectively and systematically defining, retrieving, evaluating and summarizing evidence across an entire research field. It combines systematic and scoping reviewing methods to describe a broad field of interest in terms of volume, nature and characteristics of relevant knowledge’ [Reference Catts50]. Scoping studies differ from SRs in that the former are primarily used to address broader, more complex questions, can incorporate various types of literature from formal scientific publications to informal commentaries, and methodological assessment of included studies is conducted to a limited extent or not at all [Reference Davis, Drey and Gould47]. As such, these methods are also complementary to more formal in-depth SR-MAs, which could be conducted on various focused questions of higher priority, resulting in in-depth methodological soundness assessment and formal quantitative synthesis, whenever possible and appropriate, through MA [Reference Borenstein26]. Together, these methods were also very effective at clearly outlining the problems within the existing literature and highlighted areas of focus where improvements to the global knowledge base of Salmonella in broiler chickens can be made.
CONCLUSION
The use of SR-MA outputs as model inputs provided a structured, transparent and repeatable means of obtaining and synthesizing data for use in a QEA as part of a QMRA. Despite the extensive search employed as part of the larger ScS study, there was a lack of quantitative data describing the changes in Salmonella c.f.u./carcass at the farm and during the scalding and defeathering processes. This, however, did not detract from the model's utility as a tool for evaluating different intervention strategies against Salmonella in broiler chickens from the grow-out farm to secondary processing. A combination of both farm and processing interventions was estimated to have the largest reduction in Salmonella c.f.u./carcass and prevalence by the end of chilling. The contamination entering the defeathering machine and scalding, post-evisceration washing and chilling steps of production had a large impact on the final estimated c.f.u./carcass and prevalence of Salmonella after chilling, under the current model assumptions, but may differ given a better understanding of the quantitative changes in Salmonella on-farm and during transport and defeathering.
ACKNOWLEDGEMENTS
The authors thank the Ontario Ministry of Agriculture Food and Rural Affairs, the Poultry Industry Council and the Public Health Agency of Canada for funding. We also thank Dr Hart Bailey, Lars Plym Forshell, Geoff Mead and Sue Reynolds for their expert contributions and grey literature searching. We also acknowledge Dr Sarah Totton, Lisa Waddell, Dr Wendy Wilkins, Dr Barbara Wilhelm, Janet Harris, Kyle Burgers, Vi Nguyen, Nanky Rai, Dr Lea Nogueira Borden, Heather Desjardins, Dr Abdulvahab Farzan, Judy Greig, Dr Sarah Parker, Dr Juliana Ruzante, Dr America Mederos Silveira, Dr Csaba Varga, Dr Lee Wisener, and Dr Ian Young for their assistance in conducting the systematic review and Dr Javier Sanchez, Dr Julian Higgins and William Sears for their epidemiological and statistical advice.
DECLARATION OF INTEREST
None.












