INTRODUCTION
Nipah virus (NiV) first emerged in 1998 in Ipoh, Peninsular Malaysia, causing an epidemic of fatal encephalitis in humans having a history of direct contact with infected pigs. Epidemiological evidence suggested a link between the infections in pigs, to the exposure to fruit bats attracted to the orchards surrounding the pig farm [Reference Chua1]. The link was later supported by serological evidence for NiV in five species of Malaysian bats, including the two Pteropus spp. native to Peninsular Malaysia, P. vampyrus and P. hypomelanus [Reference Johara2]. Bats have been suggested as the drivers and reservoirs of many viral zoonoses and diseases [Reference Wim3, Reference Sulkin, Allen and Melnick4]. Beyond Malaysia, India and Bangladesh where outbreaks of Nipah disease have occurred, serological evidence of NiV in pteropid bats has been found in areas with no history of Nipah disease, e.g. Cambodia, Indonesia, Thailand and Madagascar [Reference Reynes5–Reference Iehle8]. Further, antibodies to henipaviruses have been detected in non-pteropid bats in Ghana and China [Reference Hayman9, Reference Li10].
The transmission mechanism and maintenance of NiV within the Pteropus population or colony has yet to be elucidated. However, studies of Hendra virus (HeV) and NiV in bats suggest that henipaviruses can be excreted in urine, saliva [Reference Middleton11, Reference Williamson12], or by direct contact with reproductive fluids [Reference Halpin13]. Given the intense social interactions between bats in a population, it appears very likely that horizontal transmission via these secretions is one of the modes of NiV transmission. In Malaysia, NiV has only been isolated from pooled urine of P. hypomelanus [Reference Chua14] that usually roost on islands surrounding the peninsula. These bats are capable of short-distance flying and therefore, have a limited geographical distribution [Reference Jones and Kunz15]. P. hypomelanus were not found at sites where pigs and humans were infected during the outbreak periods of 1998–1999 in Malaysia. Only P. vampyrus, which has a wider geographical distribution, was recorded [Reference Epstein16].
Based on evidence of the high serological prevalence of NiV in P. vampyrus in Malaysia [Reference Sohayati17], we conducted a longitudinal study of captive P. vampyrus bats. In this study we provide evidence that naturally infected P. vampyrus bats (known by their seropositive status due to NiV infection in the wild) can harbour NiV until a time (e.g. when induced by stressed or other unknown factors) conducive enough to trigger viral recrudescence, and transmit the virus to other susceptible bats. This paper aims to describe the transmission dynamics of NiV among Pteropus bats within a population and the serological patterns that lead to virus isolation.
MATERIALS AND METHODS
Study design
We conducted a longitudinal cohort study between 12 June 2004 and 8 June 2005 on a group of 17 P. vampyrus bats whose history of exposure to NiV was unknown. On entry into the study the bats were screened for neutralizing antibodies to NiV using a serum neutralization test (SNT) and were categorized as seronegative (lack of NiV neutralizing antibodies, titre ⩽4) or seropositive (presence of NiV neutralizing antibodies, titre ⩾8). We monitored the bats for changes in NiV excretion and antibody titre for between 5 and 12 months. The Wildlife Trust Institution Animal Care and Use Committee and Department of Wildlife Malaysia (PERHILITAN) granted approval for the study project.
Study location and sample size
Seventeen P. vampyrus bats were caught from the wild using a convenient (non-random) sampling method in Lenggong (5° 07′ 01.1 N, 100° 58′ 32.7 E) and Kampung Gajah (4° 10′ 35 N, 100° 55′37 E) and all were enrolled into the study. The bats were trapped using mist nets during flying out for feeding. Two pups (n=2) born in captivity during the study period were also included in the study, making a total of 19 bats.
Each bat was marked with an ID tag and kept in the quarantine station at Taiping Zoo in Perak. Throughout the study period, the bats were housed together in a metal cage (5 m long×4 m wide×3 m height) which was roofed and isolated from human traffic. The cage was lined with a 1 cm2 wire net wall and top to minimize the chance of other Pteropus bats gaining access to the enclosure. The bats received fresh local fruits twice a day, i.e. in the morning and afternoon. Table 1 shows the rolling enrolment of the 19 P. vampyrus bats into the study.
Table 1. The ID numbers and characteristics of 19 P. vampyrus bats in captivity at entry point and the total weeks-at-riskFootnote * for Nipah virus (NiV) infection (screened between June 2004 and March 2005)
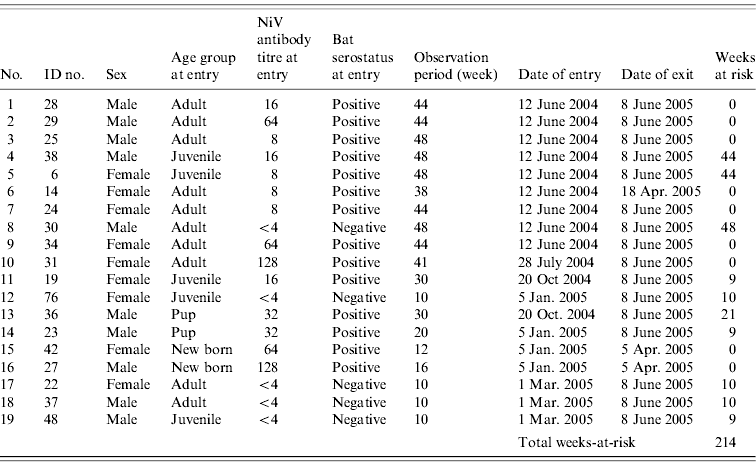
Incidence rate=2/214=9·35/1000 bat-weeks (95% CI 1·1–33 ·4/1000 bat-weeks or 57·2–1737 /1000 bat-years).
* Calculation of weeks-at-risk assumed (1) for adults, once exposed (i.e. seropositive at entry or seroconverted during the study) individuals would confer a long-lasting immunity; (2) for juveniles, once the lingering maternal antibody diminished, they are at risk for NiV infection.
Data and sample collection
Biological data recorded included five host variables (bat ID number, sex, date of enrolment, age at enrolment and reproductive status of adult female) and three sample variables (date of sample collected, neutralizing antibody titre to NiV and NiV isolation results).
According to age, we categorized each bat as either adult, juvenile or pup. A pup was defined as a young bat dependent upon and attached to its dam. An adult male was differentiated from a juvenile male based on the appearance of secondary sexual characteristics (spines on the spinosa areas of baculum). Adult females were characterized by the elongation of the nipples, which indicated prior nursing of a pup [Reference Epstein16]. We categorized the reproductive status of adult females as either ‘pregnant’, ‘carrying’, ‘nursing’ or ‘dry’. ‘Pregnant’ females were diagnosed by manual examination and positive pregnancy was determined by palpation of a fetus; ‘carrying’ females as those with an attached pup;‘nursing’ females were without a dependent pup but with active mammary glands (evident by excretion of milk on manual expression); and ‘dry’ females were adult females in none of the other categories.
We collected samples of blood, urine and saliva/throat swabs from each bat at the beginning of the study, every 7–21 days between 12 June 2004 and 29 March 2005, and every week after 29 March 2005 to 8 June 2005. All bats were anaesthetized during sample collection using ketamine and xylazine at a dose of 10 mg and 2 mg per animal, respectively [Reference Sohayati18].
Blood (plasma) samples
One ml of blood was withdrawn from the cephalic vein using a 1-inch, 22-gauge needle, and poured into a heparin tube (Meus, Italy), labelled with the bat's ID number. The sample was gently mixed by inverting the tube a few times prior to storing at ambient temperature. Two to three hours after blood collection, 0·5 ml of plasma sample was withdrawn from the blood (sediment) sample and placed in a labelled, clean cyrovial for serological (antibody quantitave) analysis.
Plasma and whole-blood cell samples
A total of 100 μl of the upper layer of blood sediment and 0·1 ml plasma in the tube was withdrawn and mixed with 0·8 ml viral transport media [VTM; Hank's balanced salt solution containing 5% FCS, 5% glycerol, amphotericin B (15 μg/ml) and penicillin G (100 U/ml), and streptomycin (50 μg/ml)], to provide 1/10 of the blood cells per sample for virological analysis.
Urine samples
We collected urine samples using a ‘free catch’ method, applying continuous light pressure on the bladder to promote urine release and collecting midstream urine using a sterile universal bottle. One ml of urine was mixed with VTM in a labelled cyrovial to give a final volume of 1/2 urine sample. If the bat failed to urinate, a urogenital tract swab (external urethral orifice in males or the vagina in females) was obtained and placed in a tube containing 0·9 ml VTM.
Saliva and/or throat samples
We collected saliva and/or throat swabs using a sterile cotton swab, which was inserted into the oropharynx and rotated gently. The swab was then immersed in a vial containing 0·9 ml VTM. All tubes containing samples were stored at 4°C prior to transferring to the Veterinary Reseach Institute (VRI), Ipoh within 3 h. Upon arrival, the samples were stored at −80°C before further analysis (within 1–2 weeks).
Virus isolation
We performed the initial virus isolation work in a Biosafety Level (BSL) 2 laboratory at VRI. The laboratory was equipped with a Class 2 Biosafety cabinet (BSC) and during the procedure laboratory personnel wore, gloves, goggles and respiratory mask, e.g. N95. All samples of throat swab, urine and whole blood in VTM were vortexed for 10–30 s before centrifugation (at 3000 g, 4°C for 10 min). Next, 200 μl of each inoculum was transferred into each well of a 24-well tissue culture plate pre-seeded with around 1×105 Vero cells (ATTC: CCL-81) and RK cells (ATTC: CCL-37) for 1 h absorption at 37°C in 5% CO2. Each of the inoculated wells was maintained with 1 ml of Eagle's minimal essential medium containing l-glutamine (Gibco, USA) supplemented with 50 mm Hepes buffer, 1000 U/ml penicillin, 1000 mg/ml streptomycin, 500 mg/ml kanamycin, 15 μg/ml amphotericin B and 2% FBS. The plates were sealed carefully with a microtitre plate sealer (Nunc, USA) and incubated at 37°C and 5% CO2. The plates were observed for cytophatic effect (CPE) daily for at least 14–15 days in three passages before the samples were declared negative. Any sample showing CPE was brought to a BSL 3 laboratory, for further viral propagation in a 25 cm2 flask of confluent Vero cells. Under the Malaysian Government Act on Control and Prevention of Infectious Diseases 1988, revised 25 May 2006, NiV is categorized in Risk Group 3, thereby allowing the virus to be handled in a BSL 3 facility.
Viral identification
Identification and confirmation of the NiV genome was performed using reverse-transcriptase (RT)–PCR. About 250 μl of tissue culture fluid was used for nucleic acid extraction using Tri Reagent (MRC, USA). A one-step RT–PCR (Promega Corp., USA) was performed using 50-μl mixture containing nuclease-free water, using a set of primers flanking 300 bp of the N gene of NiV (forward primer sequence: 5′-GCAGGAAGGCAAGAGAGTAATG-3′ and reverse primer sequence: 5′-AGGTCATTTTGAGCAGGTTTG-3′). The mixture was incubated at 50°C for 30 min followed by avian myeloblastosis virus (AMV) inactivation at 2 min at 94°C, then subjected to 30 cycles of 1 min at 94°C, 1 min at 52°C and 1 min at 72°C, followed by a final 1 min at 72°C as described previously [Reference Maizan, Mohd Ali and Sharifah19].
Antibody qualitative analysis
For all plasma samples, specific antibodies titres to NiV were determined using SNT with standardized positive plasma control. Plasma samples were inactivated at 56°C for 30 min prior to twofold dilution from 1/4 to 1/1024 in a 96-well plate. The diluted plasma samples were mixed with an equal volume of 200 tissue culture infection dose 50% (TCID50) of the virus in each well in a BSL 3 laboratory. Next, 50 μl of growth medium containing 2×104 Vero cell suspension was added to each well, making the final volume of each well 100 μl. Plates were incubated for 3–4 days at 37°C and 5% CO2 as described previously [Reference Daniels, Ksiazek and Eaton20]. Before and after each assay, the Class 3 BSC was disinfected using 70% alcohol followed by 30 min of ultraviolet light to prevent contamination on each procedure.
Antibody titres ⩾8 were determined as the cut-off titre for NiV-specific antibodies. An increase of at least fourfold in antibody titre (e.g. from 4 to 16 within a week) between serial SNTs was interpreted as an indication of acute or recent infection [Reference Thrusfield21].
Data analysis
The seroprevalence to NiV was estimated by the number of seropositive bats out of the total number of bats examined, and reported as percentage (%). The incidence rate for NiV infection was calculated by the total number of bats that seroconverted to the total time (in weeks) contributed by each bat-at-risk. The differences in the distribution of the antibody titre between sex and age at entry point were tested using the Mann–Whitney U test, W (two-group tested) at α=0·05. We examined the correlation between dam and pup antibody titre using Kendall's tau rank correlation coefficient. Analyses were conducted using SPSS v. 16 statistical software (SPSS Inc., USA).
RESULTS
The characteristics and serostatus of all 19 bats enrolled in the study are given in Table 1. Fourteen (74%) of these were NiV seropositive at entry and five (26%) were seronegative. The antibody titre of bats ranged from <4 (negative) to 128. The antibody titre of pup (n=4), juvenile (n=5) and adult (n=10) bats ranged from 32–128, 8–16 and 8–128, respectively. The difference in titre distribution in male and female bats, and among the age groups was not significant (Mann–Whitney U test, P>0·05). Bats were defined as having an infection or recrudescent episode only if the antibody titre increased by at least fourfold [Reference Thrusfield21].
Antibody status dynamics
Twelve of the 19 bats in the study were monitored for ⩾6 months and the other seven bats were monitored for <6 months. During the study period, we observed five general categories of antibody status dynamics (Table 2).
(1) ‘High positive’
Two adult female bats (ID nos. 31, 34) carrying a pup on entry, maintained high antibody titres over the study period: bat no. 31 had titres between 128 and 256 for ⩾10 months, with a mode of 128; bat no. 34 had titres fluctuating between 64 and 256, with a mode of 64.
(2) ‘Low positive’
Four adult bats (one female and three males) were in this category (bat nos. 14, 25, 28, 29). Antibody titres persisted at low levels: between 8 and 16 (bat nos. 25, 28) and 16 to 32 (bat no. 29) for ⩾11 months with a mode of 8 and 16, respectively. The other female bat (no. 14) that was pregnant (gave birth on 5 January 2005, newborn ID no. 42) and had an attached pup on entry into the study had a titre between 8 and 64 for 9 months.
(3) ‘Waning’
Two female juvenile bats (nos. 6, 19), two male pups (nos. 23, 36) still maternally dependent, and two newborn pups (nos. 27, 42) were seropositive on entry or on first bleed. Bat no. 6 was seropositive on the first bleed but within a month became seronegative. The other juvenile and two pups (nos. 19, 23, 36) were positive (antibody titre ranged from 8 to 32) on the first two and three bleeds then became seronegative from 35 week of the study. The newborn pups (nos. 27, 42) born to seropositive dams in captivity were positive for 12 and 16 weeks before the titre declined to 8, after which the pups died for reasons that were not determined.
(4) ‘Decreasing then increasing’
One adult ‘dry’ female (no. 24) and one juvenile male (no. 38) were in the first group of bats enrolled for the study and were initially seropositive when tested on entry. Within 1 and 2 months, respectively, they became seronegative. The seronegativity of these two bats continued for 10–11 months after which the antibody gradually increased from a titre of 4 up to 32 within 2 weeks (between 24 May–8 June 2005) for bat no. 24, and from negative (<4) to a titre of 16 within a week (between 31 May and 8 June 2005) for bat no. 38. Another juvenile male bat (no. 48) that was initially seronegative was also placed in this ‘increasing’ pattern because within a week (31 May–8 June 2005) it had demonstrated a fourfold rise in antibody titre.
(5) ‘Negative’
Four bats maintained a seronegative titre (<4) throughout the study period. Of these, three were adults (one male, two females).
Table 2. Serological profile: serial antibody titres of bats over a minimum 6-month period
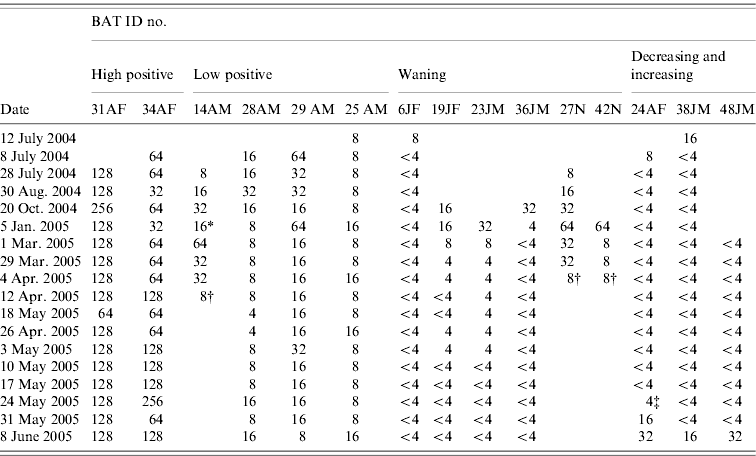
A, Adult; J, juvenile; F, female; M, male; N, newborn.
SNT titre: <4, negative; 4, doubtful; 8–32, low positive; ⩾64, high positive.
* Gave birth; † animal died; ‡ date of isolation.
Seroprevalence of dams with attached pup at entry and in dams that gave birth in captivity
Two dams with attached pups on entry were seropositive to NiV antibody (Table 3). The titre of one of the dams (no. 34) maintained at 64 (within twofold fluctuation, 32 and 128) for 32 weeks before the antibody titre increased to 128 and remained at that level for 5 weeks. Another dam (no. 14) with an attached pup (no. 23) at entry was also pregnant, and had an antibody titre of 8 on entry. The antibody titre gradually increased from 8 to 64 within 11 weeks during nursing and pregnancy. After giving birth to pup no. 42, the antibody titre of the dam waned from 64 to 8 within 12 weeks. Another dam (no. 31) that gave birth during the study was also seropositive to NiV antibody. The titre remained at level 128 with twofold fluctuations during pregnancy, nursing and dry period.
Table 3. Serial antibody titre of two pairs of dams with pups attached at the entry point of study and two dams with pups born in captivity between June 2004 and June 2005
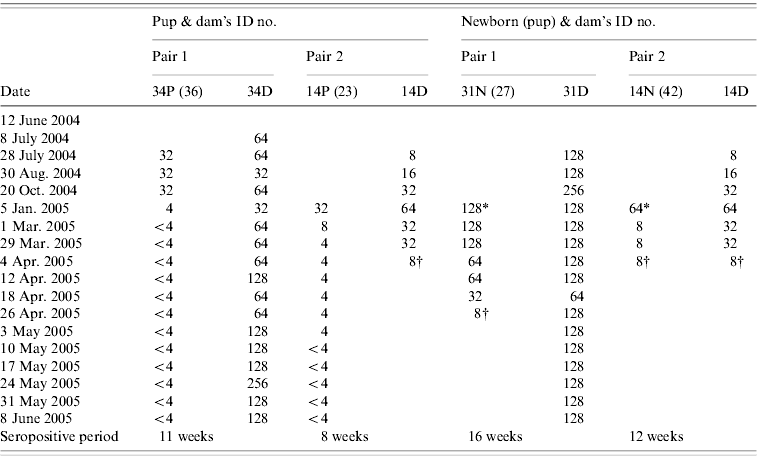
P, Pup attached to dam at entry point; N, newborn pup; D, dam.
SNT titre: <4, negative; 4, doubtful; 8–32 , low positive; ⩾64, high positive.
* Birth; † animal died.
Serostatus of pups attached to their dams on entry and pups born in captivity
We estimated two pups enrolled at the beginning of the study to be aged 4–6 months. All pups born or attached (n=4) to seropositive dams tested positive for NiV antibody (Table 3). Generally, the pups' antibody titres to NiV correlated with their dams (Kendall's tau-b test at P=0·05). However, as the samples size is small, interpretation of this result should be made with caution. The pups maintained low antibody titres ranging between 8 and 32. The titres persisted up to 35 weeks after entry into the study. The antibody titres of all pups that later became juvenile (n=2) dropped to negative (<4) between November and March, when they were estimated to be aged 9–14 months (Table 3). The antibody titres of newborns (n=2) ranged from 16 to 128 and persisted for 12–16 weeks before declining to a titre of 8, after which both pups died (pup ID nos. 27, 42). Necropsies were not performed on these pups.
Virus isolation and detection
We collected 816 specimens (272 blood samples, 272 throat swabs and 272 uro-genital swabs or urine samples) for analysis during the study period. Isolation was attempted from all samples, but NiV was isolated only from one urine sample, taken on 25 May 2005 from an adult female bat (no. 24). The virus titre in the original sample was ~101 TCID50/ml urine. Foci of NiV-like CPE was detected at day 3 post-inoculation in Vero and RK cells. The presence of NiV in the sample was confirmed by RT–PCR; however, virus was not detected from the samples of throat and blood taken on the same sampling day from the same bat. Molecular characterization and phylogenetic analysis demonstrated that this isolate is divergent from the NiV isolated from humans, pigs, and P. hypomelanus in Malaysia and more closely related to NiV reported from P. lylei in Cambodia. Details of the characterization and phylogenetic analysis are discussed elsewhere [Reference Sohayati22].
The virus was isolated from the ‘decreasing then increasing’ antibody status bat at the point when the antibody was just starting to increase (titre=4) (Fig. 1). No virus was isolated from the other two (seronegative) bats that seroconverted 2 weeks after the first isolation. At the end of the study, five seropositive (excluding the seroconverted) bats were euthanized. NiV was not isolated from the blood, brain, heart, liver, lung, kidney, spleen and uterus or testes by cell culture.
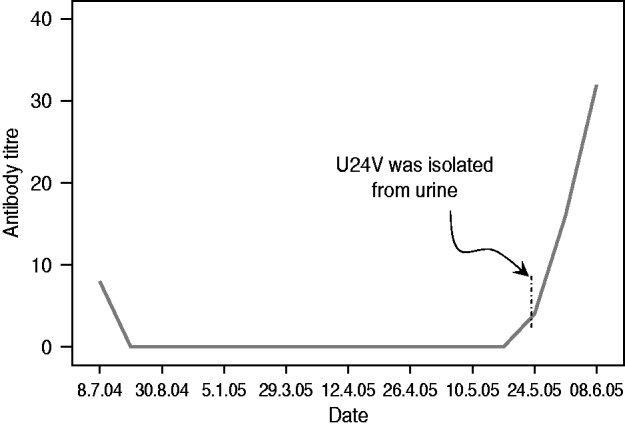
Fig. 1. Serial antibody titre of bat no. 24 that excreted Nipah virus (NiV vampyrus) in its urine on 24 May 2005 [Reference Sohayati22].
Incidence rate
In order to estimate the incidence rate (density) of NiV infection, we made two assumptions: (1) exposed and seropositive adult bats have a long-lasting immunity towards NiV; and (2) antibody titres detected on entry for juvenile bats (as well as in pups) are derived from maternal antibodies. Therefore, once the juvenile/pups become seronegative, they are then susceptible to NiV infection. Based on these two assumptions, the rate of seroconversion in this colony was estimated to be 9·35/1000 bat-weeks or 486/1000 bat-years (95% CI 1·1–33·4/1000 bat-weeks or 57·2–1737/1000 bat-years) (Table 1).
DISCUSSION
In the ‘Decreasing then increasing’ category, the adult ‘dry’ female (no. 24) excreted the virus during the week its neutralizing antibody titre increased from a titre of <4 to a titre of 4, i.e. at the initial phase of rise in titre. The virus was confirmed as NiV and the genetic characterization of the excreted virus has been fully described elsewhere [Reference Sohayati22]. Within the next 2 weeks, the bat's titre rose fourfold (titre=32), which is consistent with immunological response to an infection [Reference Thrusfield21]. We isolated NiV only from the urine of this single bat (on 25 May) despite attempts on all samples from bats in the study. Bat no. 24 appeared healthy and did not exhibit any noticeable clinical illnesses throughout the study period consistent with Middleton et al.'s [Reference Middleton11] report on the absence of clinical signs in experimentally infected bats. However, it is important to note some limitations, i.e. that the number of individuals in our study (n=19) and that of Middleton et al. [Reference Middleton11] (n=5) were low, and that we did not measure any physiological indices for health (temperature, etc.). Therefore, generalization of the findings from our study to wild bat populations should be made with caution. We suspect recrudescence in this case was triggered by an increased level of stress due to a combination of factors such as confinement in the cage, and physiological and behavioural changes during the breeding season. Stress has been shown to play an important role in lowering or suppressing immunity, thus triggering the re-activation of latent infection [Reference Bruggeman23]. Recrudescence is a known phenomenon of other paramyxoviruses such as measles and wild-type canine distemper viruses, which exhibit viral persistence in the form of a chronic progressive disease of subacute sclerosing panencephalitis [Reference Billeter24] and canine distemper [Reference Stettler25], respectively. Our finding is further supported by the evidence of neurological relapse of NiV in patients with late-onset encephalitis that were infected with NiV during the 1998–1999 outbreak [Reference Goh26, Reference Sarji27] and delayed fatal reactivation after HeV infection [Reference O'Sullivan28]. Unfortunately, bat no. 24 was released into the wild before the results of the serology and isolation were known, therefore the bat could not be further sampled and studied.
Within 2 weeks after virus isolation, two male bats (nos. 38, 48) seroconverted and demonstrated a fourfold increase in the antibody titre. Temporal seroconversion clustering of the three bats in this colony is consistent with a recent viral challenge and a scenario that bat no. 24 underwent recrudescence of a latent infection and bat nos. 38 and 48 contracted the infection horizontally through exposure to virus secreted by bat no. 24. Studies of HeV and NiV in bats suggest that henipaviruses can be excreted in urine, saliva [Reference Middleton11, Reference Williamson12], or by direct contact with reproductive fluids [Reference Halpin13]. Our supposition fits the regular grooming behaviour and the biting/licking behaviour of bats in the wild prior to mating [Reference Hall and Richards29], which, in this study increased the likelihood of horizontal viral transmission via the aforementioned secretions. Our findings support Plowright et al. [Reference Plowright30] who emphasized the importance of horizontal transmission for HeV within a Pteropus bat community. Nonetheless, we were unable to explain why NiV was not isolated from any samples derived from the two seroconverted male bats. We speculate that the viraemic period of NiV in infected bats is narrow, or that the virus resides in certain organs or cells rendering it unavailable in blood. Furthermore, the short viraemic phase or a short excretion period may have been missed due to the gaps between samplings. Findings in experimental infections where NiV was found in urine between 12 and 18 days post-infection [Reference Middleton11], and HeV in blood at 10 days post-infection [Reference Field31] appear to be consistent with our claims. We also discovered that NiV was excreted in the urine at very small quantities (~101 TCID50), which additionally decreases the likelihood of virus isolation.
In our study, two groups of bats were at risk for NiV infection, adults and juveniles. In adults, we considered (based on anology of a few other paramyxoviruses such as wild-type canine distemper and measles of the genus Morbillivirus) that once exposed (i.e. seropositive at entry or seroconverted during the study) individuals would confer a long-lasting immunity. In juveniles, we assumed that once the lingering maternal antibody diminished, these bats would be at risk for NiV infection. Based on these assumptions, we deduced that the incidence of seroconversion (negative to positive) in the colony was high (9·35/1000 bat-weeks or 486/1000 bat-years). This translates into an average time of 2·06 years or 24·7 months before a bat became infected in this population. The high incidence rate in this study and high seroprevalence of P. vampyrus and P. hypomelanus in Peninsular Malaysia (32·5% and 11%, respectively) [Reference Sohayati17] suggests a high risk of an individual bats acquiring NiV in the wild.
The rate of seroconversion in this colony might be explained by the interaction among three important factors: (i) characteristics of hosts; (ii) characteristics of pathogens; and (iii) effective contact. A host's infectiousness determines its ability to transmit an infection. Individuals of Pteropus in a colony (in the wild or in captivity) generally interact intensively [Reference Hall and Richards29], which greatly enhances viral transmission if an infectious host is present. Assuming that mixing among bats in the small colony we managed here is random then every susceptible individual would have an equal chance of being exposed to the virus if just one individual is shedding. However, as the bats were studied in a captive environment, the observations we recorded may have to be interpreted with caution as situations in the wild may differ (e.g. open population) and result in a different rate of viral transmission.
The serological profile of dams and their pups and newborns in this study suggests that maternal antibodies were transferred from dam to pup. The antibody titre to NiV in newborn pups with seropositive dams persisted for at least 4 months (16 weeks) before it waned (it may have reached beyond, but the newborn died) and in dependent pup or juvenile bats, up to an additional 6 months into the study before they became and remained seronegative. Our observation is consistent with the findings of Plowright et al. [Reference Plowright30] on the age-specific waning of HeV seroprevalence among Pteropus pup and juveniles. Furthermore, Hall & Richards [Reference Hall and Richards29] described lactation lasting up to several months in captivity or up to 6 years in semi-captive bat populations while Field [Reference Field31] suggested that the HeV antibody in Pteropus pups lasts for 7 months.
Our data finally demonstrate that the only bat species identified at the farm, i.e. P. vampyrus where the index case for NiV occurred in Ipoh, Malaysia, during the outbreak, are able to carry NiV. Evidence for NiV recrudescence significantly changes our understanding of henipavirus circulation in natural reservoir hosts and, potentially, domestic animal hosts. Approaches used to model henipaviruses thus far have used SEIR models that have assumed a short infectious period and long-term immunity [Reference Plowright30]. Repeated viral shedding by an individual over time will change these models substantially. For example, SEIR models suggested that migration of infected bats from other colonies, via metapopulation dynamics, is necessary for maintenance of henipaviruses in Pteropus colonies [Reference Plowright30]. A model that includes recrudescence will allow viral circulation in a population without the need for migration of infected individuals. Repeated shedding of NiV may lead to a steady rate of infection in susceptible individuals in bat colonies. In addition, the virus can be maintained without the ‘boom and bust’ dynamics typical of acute viral infections that is usually associated with an increased probability of population extinction [Reference Berryman and Millstein32].
ACKNOWLEDGEMENTS
We thank Mr Azizi M. Yatim, Mr Hamdon Tak, Mr Amir Nordin Harun, Miss Norhayati M. Noor and Mr Abdul Karim Abdul Hamid for their support in the field and laboratory work. We also thank the Department of Wildlife and National Parks of Malaysia for the special permit and Zoo Taiping and Night Safari for permission to use their quarantine facilities. This study was funded by an NIH/NSF ‘Ecology of Infectious Diseases’ award from the John E. Forgarty International Center (R01-TW05869), and by the internal fund of the Veterinary Research Institute, the Malaysian Department of Veterinary Services. This paper is published in collaboration with the Australian Biosecurity Cooperative Research Center for Emerging Infectious Disease.
Dr Sohayati is a veterinarian who has conducted research on NiV in wild animals including Pteropus spp. in Peninsular Malaysia since 2000. Her interest is in the ecology of emerging and re-emerging diseases transmitted by wildlife, especially by bats and rodents.
DECLARATION OF INTEREST
None.





