Introduction
Enterovirus 71 (EV-A71) outbreaks have been raising considerable public health concerns since the late 1990s [Reference Wang1–Reference McMinn11]. EV-A71 infection may be asymptomatic of cause diarrhoea, rashes, and hand, foot and mouth disease (HFMD). These symptoms predominated in laboratory-confirmed cases, present in 45% of mild, 80% of severe and 93% of fatal cases [Reference Shih12]. EV-A71 infection is especially known for its role in epidemics of severe neurological diseases in children, sometimes leading to death [Reference Lin, Chu and Chiu13]. Indeed, the mortality rate of severe EV-A71 infection cases was 5.14% [Reference Shih12]. Fatal cases appear about 3 days from the onset of illness [Reference Ho7, Reference Xing14]. The cause of death tends to be brainstem function failure [Reference Shindarov15–Reference Yang18]. Studies have reported that most fatal cases were 5 years of age or younger, and all were with similar manifestations, i.e. autonomic nervous system failure and other neurological symptoms before death [Reference Wang1, Reference Lin2, Reference Ho7, Reference Xing14].
Autopsies and animal models indicated that the main lesions appear to be localised to the brainstem [Reference Shindarov15, Reference Lum16, Reference Yang18–Reference Li25]. Indeed, brainstem encephalitis accounts for 58% of neurological manifestations of EV-A71 infection, followed by aseptic meningitis (36%) [Reference Shindarov15, Reference Lum16, Reference Yang18–Reference Lee26]. Neurological symptoms include myoclonus, ataxia, nystagmus, oculomotor palsies and bulbar palsy, and imaging can be either positive or negative [Reference Lee26]. Receptors for EV-A71 have been identified on human cells; SCARB2 is the main receptor of EV-A71 in nerve tissue, allowing the virus release its RNA into neurones [Reference Lin2–Reference Xu5]. Nevertheless, the reasons for brainstem involvement are unknown [Reference Lee26].
In addition, there is a lack of reliable indicators for the early identification of the cases at higher risk of death. Determining the symptoms and signs that can be associated with a fatal progression of EV-A71 infection could be useful to identify the patients who could benefit from treatments such as intravenous immunoglobulins and milrinone [Reference Lee26]. Therefore, the aim of the present study was to examine the natural history of fatal EV-A71 infection and to identify the symptoms and signs for early warning of deterioration. To do so, a 5-year multicentre clinical observational study was performed.
Methods
Study design
This was a clinical observational study of fatal cases of EV-A71 infection. Among the severe cases of EV-A71 infection, we reviewed all HFMD fatal cases records to analyse the risk factors associated with mortality [Reference YY27–Reference Yang29]. Seven centres designated to treat HFMD in the coastal cities of Guangdong province participated in this study and were trained for the identification of fatal cases. The data of the fatal cases came from five centres; the other two hospitals had no fatal cases.
This study was approved by the Ethics Committee of our institution. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The legal guardians of the children provided a written informed consent to be included in our databank of patients with EV-A71 infection.
Patients
In 2008–2009, all paediatricians working at the Department of Paediatrics of the seven participating hospitals were trained to recognise the symptoms and signs of EV-A71 infection, including the first-, second- and third-line physicians. Sida Yang, the study principal investigator, also performed random inspections to assess accuracy (10% of the entries). Between 1 January 2010 and 31 December 2012, we recorded the symptoms and signs of all EV-A71 infection cases using a standard form. The manifestation, sequence and duration (in hours) of all symptoms and signs were noted.
The inclusion criteria were: (1) <14 years of age; (2) suspected EV-A71 infection (patients with HFMD or patients without rash or herpangina but with neurological symptoms); and (3) confirmed by real-time PCR using a throat swab sample, as previously described (ABI 7300 real-time fluorescence quantitative PCR; C t value <34.9 was considered positive) [Reference Piao30]. Those who met all the above whole criteria were included.
The exclusion criteria were: (1) tested positive for any pathogens other than EV-A71 (including any other enterovirus or CoxA16); (2) trauma; (3) intoxication; (4) received any vaccine within 4 weeks (to exclude patients with increased susceptibility to EV-A71 because of immune reaction to the vaccine or confounding symptoms from the vaccine); or (5) other central nervous system (CNS) diseases such as cerebral palsy or intracranial space-occupying lesion. Among 5504 confirmed severe cases of EV-A71, 54 fatal cases were identified after application of all above inclusion/exclusion criteria.
Observational indexes
Based on our previous studies [Reference YY27–Reference Yang29], we identified and defined 91 symptoms and signs of severe cases of EV-A71 infection. The study board included paediatricians and paediatric neurologists, including HFMD specialists from the National Health and Family Planning Commission of the People's Republic of China, experts from the National Clinical Research Centre for Respiratory Disease and from the Institute of Neuroscience of Guangzhou Medical University. These experts reviewed and determined all 91 symptoms and signs. All severe cases were treated according to the national guidelines (in Chinese: http://www.gov.cn/gzdt/2008-05/03/content_960347.htm; or the English version from Hong Kong: http://www.dh.gov.hk/chs/useful/useful_ld/files/ltod20070523.pdf).
The manifestations of fatal cases included general indicators (fever, rash, herpangina and flu-like symptoms), non-specific indicators (vomiting, persistent hyperpyrexia, hydrostomia, headache, dizziness and palpitation), dyskinesia (46 items), disturbance of consciousness (five items) and autonomic dysfunction (29 items) (Supplementary Material 1) [Reference Yang31]. The time from onset of disease was defined as the onset of initial symptom in the patient's medical history.
The autopsy had to be authorised by the legal guardians and was performed at the Forensic Medical Identification Centre of Sun Yat-Sen University.
Statistical analyses
Descriptive statistics were used (frequencies, mean ± s.d., median). Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA). Multilayer perceptron analysis [Reference Meng32–Reference Wei34] was used for the stable prediction of the possibility or category of death under the effects of multivariates/multicovariates [Reference Haykin35]. Briefly, the 91 indicators observed in this study were divided randomly into two groups (the training and testing groups), and then the possible associations between these indicators with death were assessed. If the percentages in the two groups are similar, the results indicate that the indicators are statistically significant in stably predicting death. This method was used to compensate the limitation of lacking controls for survival.
Results
Characteristics of the patients
Between 1 January 2010 and 31 December 2012, there were 5504 inpatients confirmed with severe EV-A71 infection in five hospitals, including 54 fatal cases (0.98%). Among the fatal cases, there were 31 males and 23 females. There were 26 cases from urban areas and 28 cases from rural areas. Median age was 21.5 months (Q1−Q3: 12–36; mean, 23.0 ± 13.8 months; 47 cases (87.04%) were 3–36 months old. The median duration from onset to death was 3.3 days (range, 0.25–18); 43 cases (79.4%) died within 5 days, and four cases died between 11 and 18 days as they received paediatric advanced life support (PALS) within 5 days after illness onset. Illness onset was defined as the appearance of the first symptom.
Occurrence time of symptoms or signs
Table 1 shows presents the 91 indicators recorded. Except fever, the most common symptom before death was pulmonary oedema and/or haemorrhage (n = 46, 85.2%), followed by capillary refill time (CRT) extension (n = 43, 79.6%). The signs/symptoms that were present in more than 50% (⩾27) of the fatal cases were fever (n = 52, 96.3%), pulmonary oedema and/or haemorrhage (n = 46, 85.2%), CRT extension (n = 43, 79.6%), tachycardia (n = 36, 66.7%), rash (n = 36, 66.7%), clammy skin (n = 32, 59.3%), vomiting (n = 31, 57.4%), hyperventilation (n = 29, 53.7%), irregular respiratory rhythm (n = 28, 51.9%) and pale skin (n = 27, 50.0%). The signs/symptoms that were present in more than 30% (⩾16) of the fatal cases were startle (n = 25, 46.3%), persistent hyperpyrexia (n = 24, 44.4%), deep coma (n = 24, 44.4%), refractory shock (n = 23, 42.6%), somnolence (n = 22, 40.7%), tachypnoea (n = 22, 40.7%), myoclonic jerks/tremors (n = 21, 38.9%), ultrahyperpyrexia (n = 20, 37.0%), anxiety (n = 19, 35.2%), mottled skin (n = 19, 35.2%), dysphoria (n = 18, 33.3%), absence of pharyngeal reflex (n = 18, 33.3%) and excessive tachycardia (n = 16, 29.6%).
Table 1. Signs and symptoms in 54 fatal cases of EV71 infection and time of occurrence (h)

Note: *Median duration of symptoms/signs occurring within 6 h before death.
**Median duration of symptoms/signs occurring within 12 h before death.
Relation between death and signs/symptoms
Three patients died within 24 h after onset, and 23 symptom or signs had been observed from the list of 91 signs/symptoms. The shortest duration from onset to death was 6 h; this case presented with fever first, clammy limbs followed, then death after from pulmonary haemorrhage. The other two cases presented ataxia respiratory or pulmonary haemorrhage; none of the three were comatose before PALS. Five cases died 1–1.5 days after onset and they showed 58 symptoms or signs. Autonomic dysfunction was found in all five cases, including poor peripheral perfusion and pulmonary oedema and/or haemorrhage. For the patients who died >1.5 days after onset, a wide diversity of manifestations could be observed. These patients deteriorated sharply after autonomic nerve failure, including refractory shock, Neurogenic pulmonary edema (NPE), excessive tachycardia, ultrahypertension and deep coma. When these five symptoms persisted for 6 h (median duration), the patients died (Fig. 1).

Fig. 1. Median duration of symptoms/signs occurring before death in patients with enterovirus A7 infection.
Multilayer perceptron analysis
The multilayer perceptron analysis showed that age and 15 symptoms and signs predicted a fatal progression. These symptoms/signs were ataxia respiratory (100% of training, 100% of testing), ultrahyperpyrexia (92.3%, 85.7%), excessive tachycardia (80.0%, 83.3%), refractory shock (75.7%, 85.0%), pharyngeal reflex absent (71.4%, 75.0%), irregular respiratory rhythm (65.0%, 73.0%), hyperventilation (66.7%, 72.7%), deep coma (60.0%, 66.7%), pulmonary oedema and/or haemorrhage (59.5%, 55.6%), excessive hypertension (50.0%, 42.9%), tachycardia (50.0%, 42.9%), somnolence (43.8%, 50.0%), CRT extension (39.3%, 33.3%), fatigue or sleepiness (34.6%, 39.1%) and age (30.0%, 35.7%) (Table 2).
Table 2. Multilayer perceptron of signs/symptoms associated with death from of non-survivors after enterovirus A71
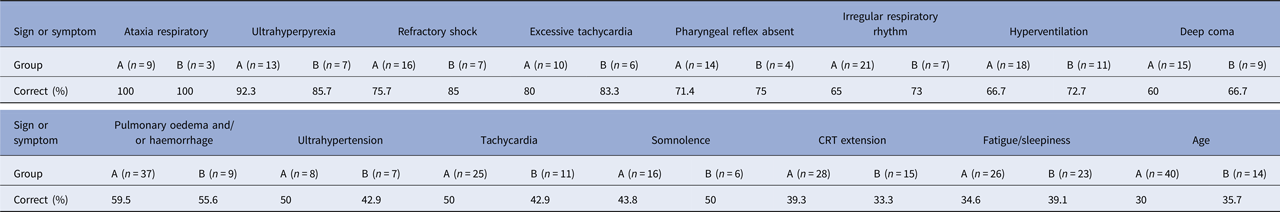
Group A: Training; Group B: Testing.
Autopsy findings
Macroscopic examination showed brainstem oedema (Fig. 2a). The brain and brainstem sections showed no bleeding or hernia. The texture of the two lungs was more solid than normal and there were foam or pale red liquid overflow in the lung sections (Fig. 2b). There were no abnormal appearance of the heart (intracardiac structure and coronary) and no bleeding in the myocardial sections (Fig. 2c).
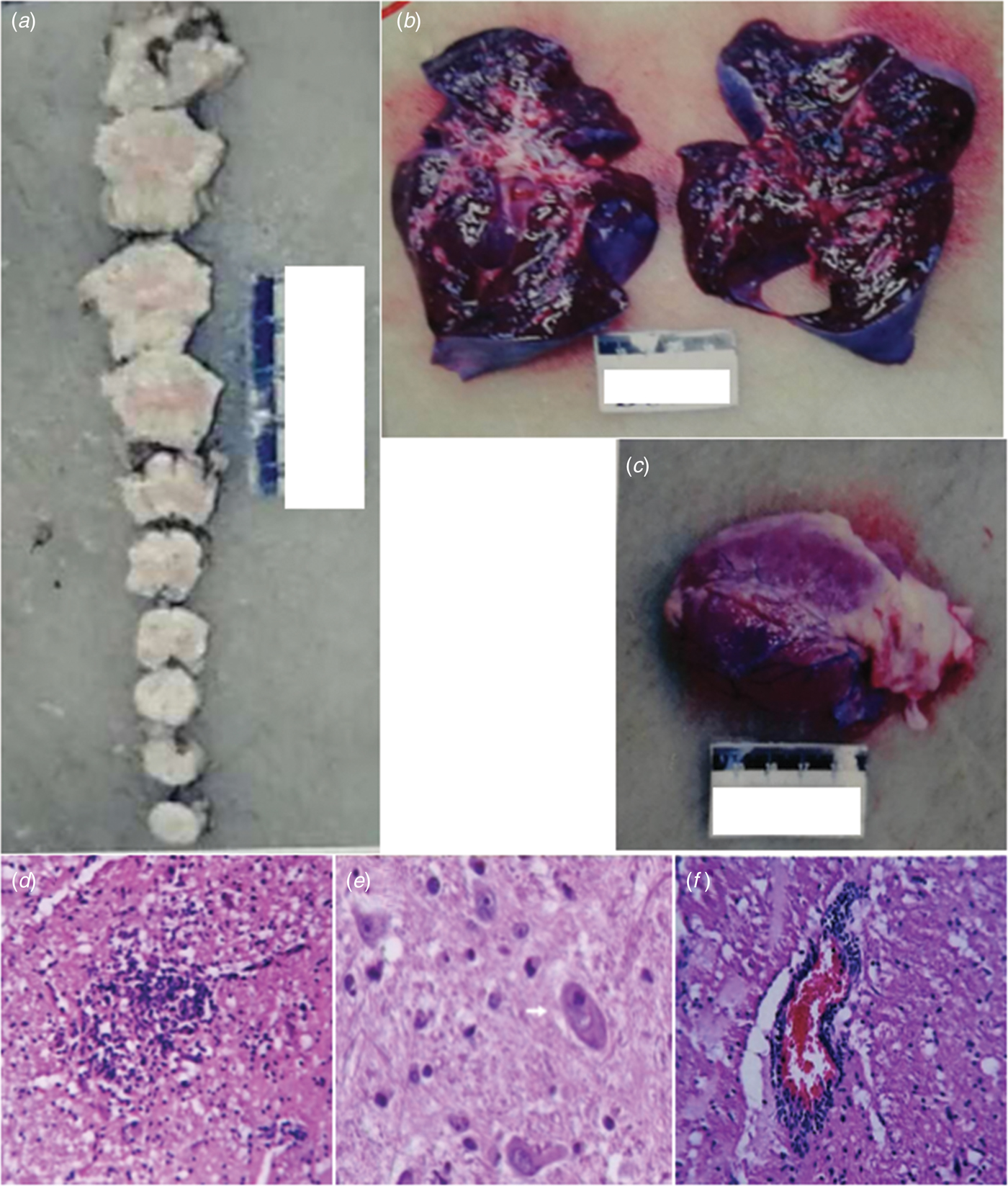
Fig. 2. Macroscopic examination of the brainstem (a), lung (b) and heart (c). Gliacyte proliferation and accumulation of tuberculum in the brainstem; vacuolar degeneration of neurons formed reticular necrosis lesion (d). Brainstem neurons showed neuronophagia in fatal cases of enterovirus A71. The pathological studies of brainstem showed neuronophagia, HE × 400 (e). Lymphocytes and small glial cell infiltration around vessels in the brainstem, showing ‘sleeve like’ change (f).
Microscopic examination of the brain showed that there was no haemorrhage in the brain parenchyma. Necrosis of the brainstem and formation of the softening spots were observed (Fig. 2d). There were microglia infiltration and the phenomenon of neuronophagia (Fig. 2e). Glial nodule was observed (Fig. 2d). Interstitial small vessels were dilated and congested and there were many lymphocytes and mononuclear cells infiltrated around the vessels, showing ‘sleeve like’ changes (Fig. 2f).
Microscopic examination of the heart, lung and other organs showed that the lungs were with alveolar wall thickening, interstitial fibrous tissue hyperplasia, vascular dilatation and hyperemia, alveolar cavity pink or pale homogeneous oedema liquid, no inflammatory cell infiltration and a small amount of transparent membrane formation. The heart showed no myocardial necrosis and inflammatory cell infiltration, interstitial mild congestion, oedema, no bleeding and myocardial fibre breakage in a wavy arrangement. There were no obvious pathological changes in the other organs (such as liver, spleen, kidney, adrenal gland, pancreas, thymus, gastrointestinal tract, etc.).
Discussion
EV-A71 infection may be fatal, but the natural history, symptoms and signs are poorly understood. Therefore, this study aimed to examine the natural history of fatal EV-A71 infection and to identify the symptoms and signs for early warning of deterioration. The results showed that all fatal cases of enterovirus A71 had neurologic involvement, even at the early stage.
Despite the fact that the mechanisms of neurological involvement in EV-A71 infections remain mostly unknown [Reference Lee26], recent advances shed some light on this point. Among the fatal cases, neurological symptoms and signs could be observed at any stage and autonomic dysfunction was the most common before death. Some symptoms observed in the present study were similar to those of other studies [Reference Wang1, Reference Lin2, Reference Ho7, Reference Xing14], but these early reports lack the timing of the manifestations. Compared with the surviving patients, the symptoms of autonomic nerve dysfunction appeared earlier in fatal cases [Reference Gao, Yang and Tao36]. The neurological involvement of the limbic system, pyramidal system, extrapyramidal and autonomic nerve was similar to that of the macaque model developed by Nagata et al. [Reference Nagata37, Reference Nagata38]. SCARB2 is essential for neurological involvement during EV-A71 infection [Reference Yu39–Reference Yamayoshi43]. EV-A71 binds to myelin SCARB2, which induces degranulation and neuron damage [Reference Lin40, Reference Yamayoshi41]. Nagata et al. [Reference Nagata37, Reference Nagata38, Reference Li44] showed in a macaque model of EV-A71 infection that the damaged neural area included limbic system, pyramidal system, extrapyramidal and autonomic nerves. By binding to SCARB2 receptors on myelin of these regions, EV-A71 can attack the brainstem within a few hours through reverse axonal transport [Reference Tan, Ong and Wong45–Reference Wang48]. Autopsy studies showed that the brain, especially the brainstem, was the most severely involved [Reference Jiang19, Reference Wong49–Reference Hsueh51]. Neurons in areas of inflammation and tissue necrosis have been shown to be positive for EV-A71 by immunohistochemistry [52]. These findings were similar to the autopsy findings of the present study, and could explain, at least in part, the neurological manifestations. Nevertheless, additional studies are necessary to determine whether myelin structures provide a direct neural pathway for EV-A71 invasion since only two patients could be examined post mortem.
In the present study, the patients of 3–36 months of age represented 87.0% of the fatal cases, which was not only similar to a large study in China [Reference Chang53], but also to a number of previous studies [Reference Ho7, Reference Xing14, Reference Chua and Kasri54]. The multilayer perceptron analysis showed that age was one of the valid factors predicting progression to death. This age distribution matches the age of active myelination [Reference Haynes55–Reference Dean60]. Nevertheless, whether the age distribution of severe vulnerability is related to active myelination [Reference Chen46, Reference Haynes55–Reference Ryniewicz58, Reference Dean60, Reference Bartkowska-Sniatkowska61] will have to be confirmed in a future study.
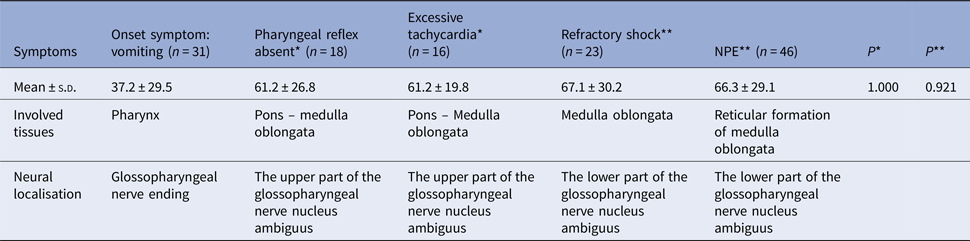
The rapid progression and the neurological pathway suggested here may be associated with the biology of EV-A71. Indeed, EV-A71 is transmitted by droplets or direct contact [Reference He17, Reference Lui62] and often invades the patients through the mucosa of the oropharynx, throat and eyes [Reference Chen63–Reference Zhang65]. Because EV-A71 has muscle- and neurotropism properties [Reference Nagata37, Reference Tan, Ong and Wong45, Reference Chen66, Reference Chen67], the neurological involvement could occur immediately after invasion. Stimulation of invasion by EV-A71 could also lead to startle being the initial symptom [Reference Herbert68, Reference Henck, Reindel and Anderson69], as we observed in 46.9% of the patients, In addition to myoclonic jerks/tremors and vomiting. The nerve conduction involved in these onset-symptoms is shown in Table 3. When eye or nasal mucosa invasion triggered visual or smell startle reflex and startle onset [Reference Lin and Shih70–Reference Liang72], then facial motor nerve involvement, triggering nerve impulse conducting to the red nucleus, and myoclonic jerks/tremors occurred, while invasion from the pharyngeal mucosa triggered vomiting (see Fig. 3). Binding with SCARB2 in myelin induces EV-A71 uncoating and cause neuronal necrosis in the brainstem [Reference He17, Reference Yamayoshi43, Reference Wong49, Reference Lin and Shih70–Reference Lu77], cervical spine cord, spinal cord ventral horn, cerebellum and interbrain central nervous tissues [Reference Yang18, Reference Huang20, Reference Tan, Ong and Wong45, Reference Hsueh51, Reference Lu77]. As autopsy reports showed [Reference Gluska78], the brainstem damage observed in the present study was similar. The damage process of EV-A71 can be seen from the symptoms of nerve involvement. In the case of glossopharyngeal nerve, we observed the damage from the peripheral nerves to the glossopharyngeal nucleus (ambiguus) which locates in the medulla oblongata, i.e. the life-centre (Table 4). The length of the cranial nerve in infants and children is similar, and there was no difference in the occurrence time of neurological symptoms caused by the same nerve structure involvement in our cases (Table 4), suggesting that a cranial nerve pathway could be existing.
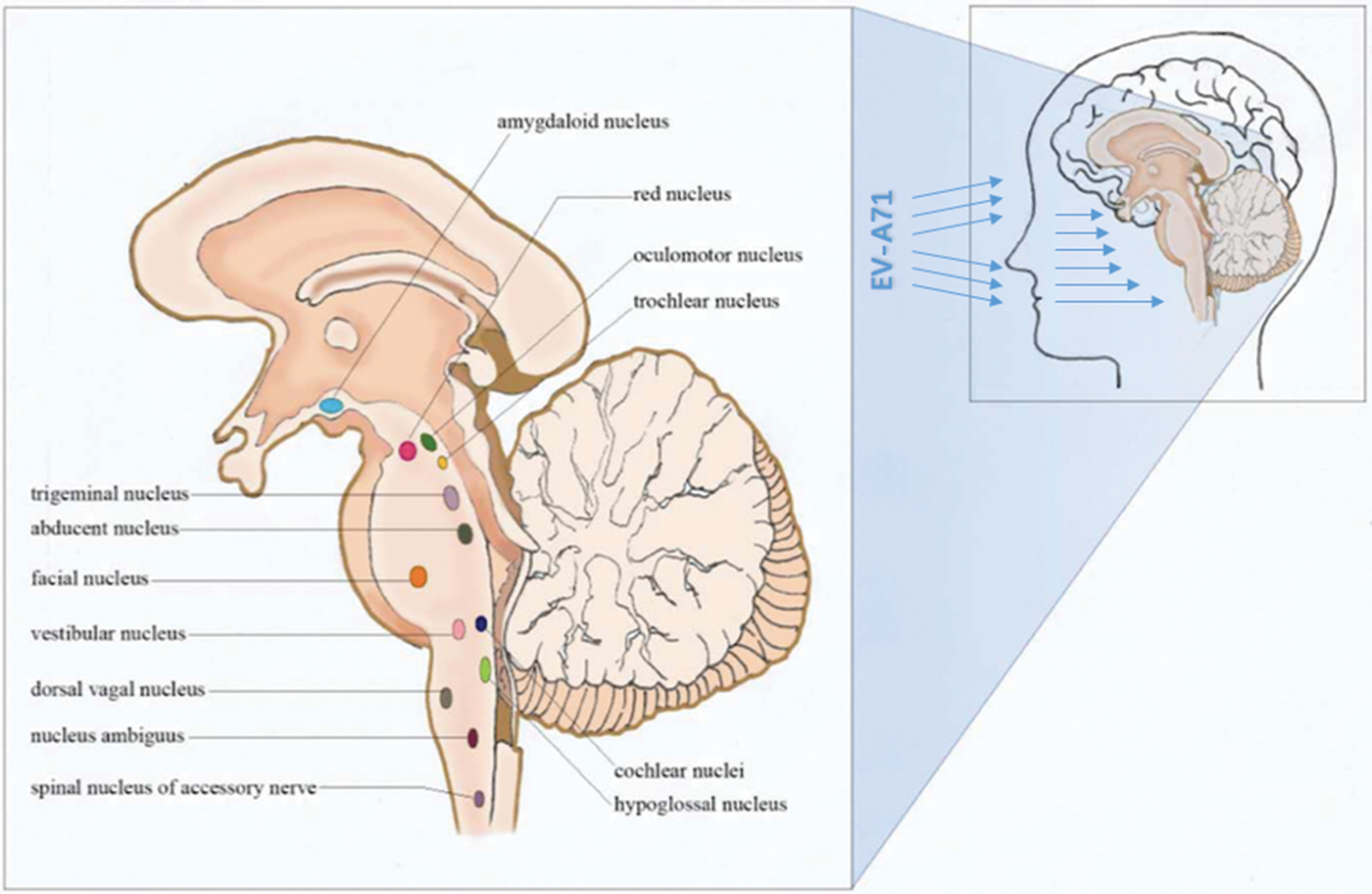
Fig. 3. Invasion entrance of enterovirus A71 (mucosa of the oropharynx, throat, nose and eyes).
Table 3. Nerve conduction involved in the onset-symptoms
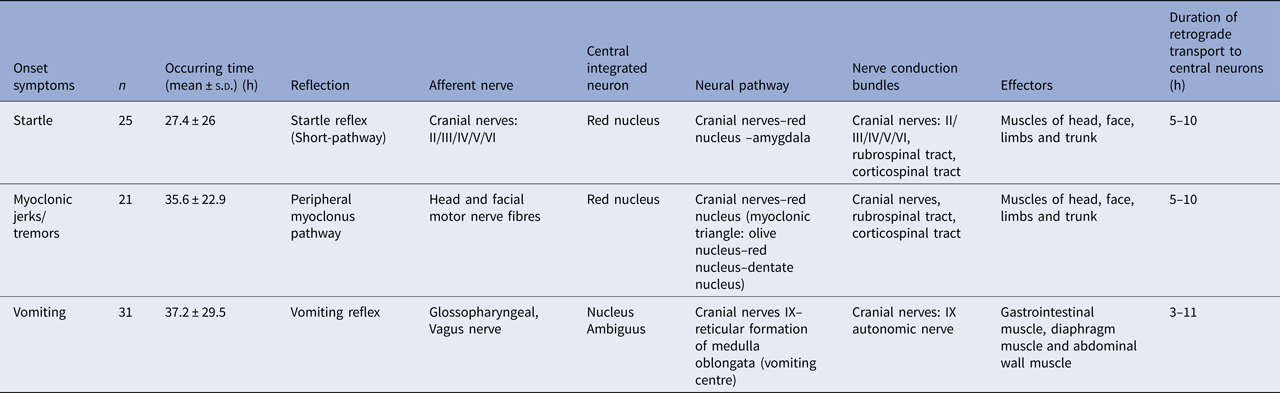
Table 4. The comparison of occurring-time of symptoms with glossopharyngeal involvement (h)
Some animal experiments and autopsy studies reported that EV-A71 was detected in the brainstem tissue [Reference Xing14, Reference Lum16, Reference He17, Reference Nagata37, 79]. EV-A71 can attack the brainstem within a few hours via motor nerves by reverse axonal transport [Reference Tan, Ong and Wong45, Reference Chen46, Reference Wong and KC75] Animal experiments showed that EV-A71 entered the brainstem through the cranial nerve [Reference Fujimoto23], which provides a basis for the existence of neural pathways in the disease. The duration from onset to death depends on the velocity of this reverse axonal transportation, the length of the cranial nerves and the characteristics of the virus including its virulence, spreading mode and transmission. Further study is needed to explore whether EV-A71 can hijack and accelerate axonal transport, as other small RNA viruses do [Reference Gluska78]. The speed of retrograde axonal transportation is 205 mm/24 h. Because the cranial nerve is shorter and the retrograde axonal transport is faster in infant and young children, the time of transportation of substances through the cranial nerve, including viruses, to the brainstem neurons is shorter (Supplementary Materials 2) Furthermore, the reticular formation of the brainstem creates conditions for the expansion of viral damage, resulting in the loss of a large number of brainstem neurons, especially the medullary neurons, leading to death. It can explain the rapid fatal process of extreme cases from onset to death after only 6 h in the present. Nevertheless, it has to be underlined that these mechanisms are hypotheses based on the literature and were not directly observed in the present study, even if they conveniently fit our observations. The related molecular biological mechanisms need to be further studied.
There are two more noticeable findings in the present study. The occurrence of unconsciousness will progress to death in the fatal cases, regardless of the duration or level of unconsciousness. This phenomenon probably just reflected that EV-A71 invades different areas of the awakening brain centres. In addition, fatigue was the only observed sign when pulmonary oedema and/or haemorrhage occurred in four patients, and this phenomenon might be related to direct attack on the medulla oblongata or spinal cord [Reference Sedy80], without attack on the awakening centre.
In summary, direct EV-A71 invasion and subsequent brainstem–neuron damage could be said to be the important causes of death in patients with EV-A71 infection. Combining with the clinical manifestation, the pathological anatomy and previously reported biology of EV-A71, we attempted to describe the neural invasion pathway of EV-A71 (Fig. 4). According to our hypothesis, EV-A71 spreads through droplets or direct contact to infect children. EV-A71 invades the mucosa of oropharynx, throat, eyes and nose (multiplication in the skeletal muscle). EV-A71 reaches the neurons through motor nerves in the muscles of the head, face and oropharynx by reverse axonal transport, and causes the relevant symptoms and signs. EV-A71 will be transmitted through the neural networks to other related CNS areas. Once EV-A71 entered the neural pathway, the distance of attacking medulla could be calculated in millimetres and the duration from onset to death could be calculated in minutes. The present study strongly suggests that it is important for clinicians to identify neurological manifestations of EV-A71. In practice, identification of severe cases will help clinicians provide appropriate assessment and intervention.
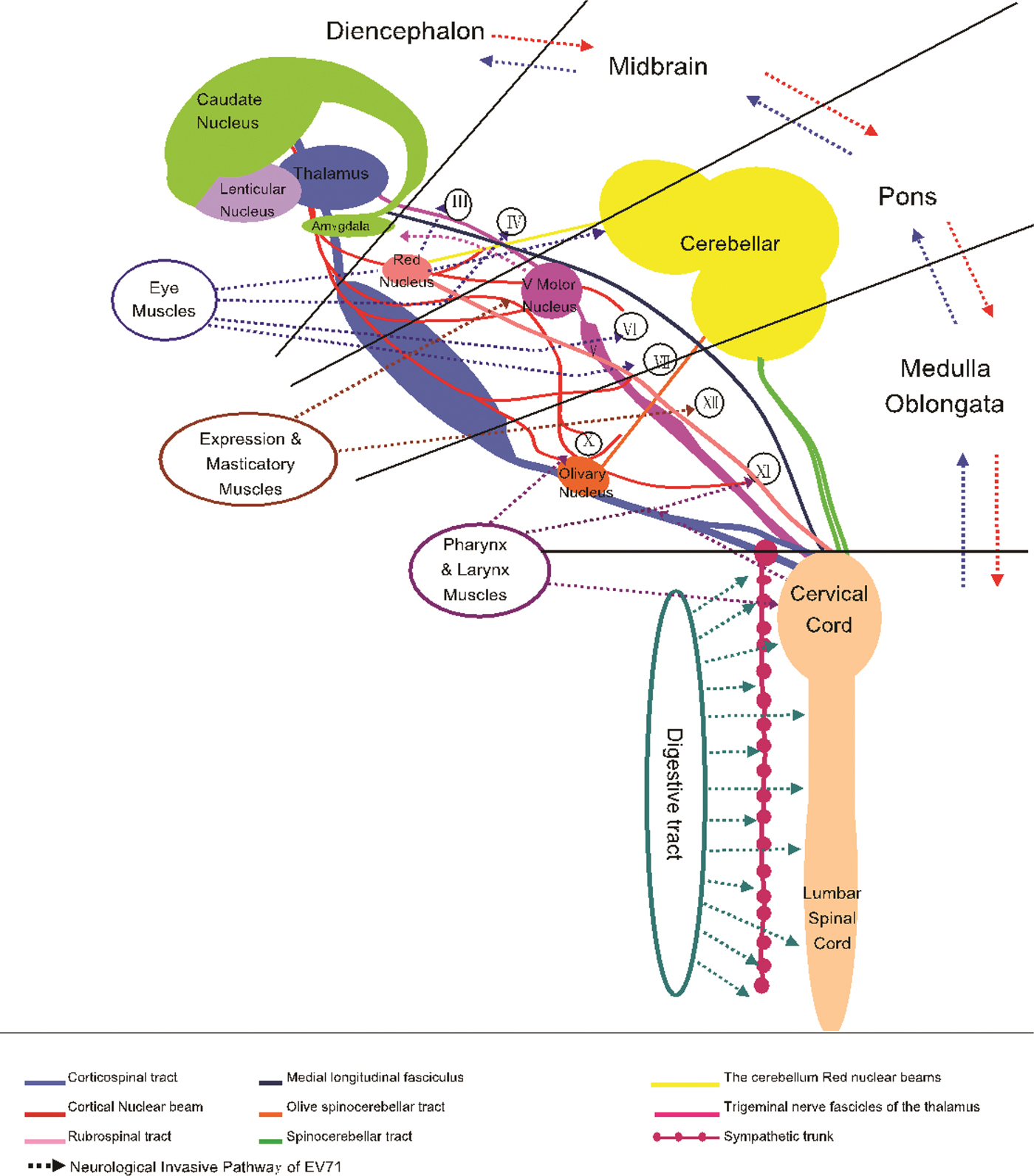
Fig. 4. Neurological invasion pathway of enterovirus A71.
The World Health Organization suggests that the clinical monitoring indicators for HFMD should include basic vital signs, myoclonic jerks, profuse sweating, central venous pressure, arterial blood gases and echocardiography [79]. In the present study, abnormalities could be observed in <50% of the cases and these patients could progress rapidly to death. In these circumstances, the cranial nerve symptoms and signs, the level of consciousness and autonomic nerve function like poor peripheral circulation should be assessed and reassessed, in order to more fully understand the degree of disease progression, then get early warning of critical state.
This study has some limitations. Firstly, the observed cases were only from coastal cities in Guangdong Province, and the sample size was small, precluding any reliable calculation of sensitivity and specificity. Therefore, the generalisability of our study might be challengeable. Secondly, we only interpreted the observation and analysis of the clinical facts. The present facts supported that neurological symptoms were valid for the early assessment of the risk of death, but the actual validity will have to be tested in a future study that will include controls. Thirdly, due to ethical reasons, autopsy could only confirm the main cause of death, and no exploratory and comprehensive autopsy could be performed. Thus, brainstem lesion could not be explained as an independent reason for death. In addition, due to failure to obtain the legal guardians’ consent, the autopsy cases were limited, and EV-A71 virus isolation or PCR was not performed due to Chinese regulations.
Conclusion
The fatal cases of enterovirus A71 had neurologic involvement, even at the early stage. Direct virus invasion through neural pathway and subsequent brainstem damage might explain the rapid progress to death.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818000468.
Acknowledgements
We thank Chongfan Zhang, the editorial director of Clin J Evid Based Pediatr, for guidance in the writing. We also thank Mingqi Zhao Ph.D. for providing professional help on pathogen detection.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interests
The authors declare that they have no conflict of interests.
Ethical standards
This study was approved by the Ethics Committee of our institutions. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The legal guardians of the children provided a written informed consent to be included in our databank of patients with EV-A71 infection. Due to failure to obtain the legal guardians’ consent, the autopsy cases were limited, and EV-A71 virus isolation or PCR was not performed.
Authors’ contributions
SDY is the guarantor for integrity of the entire study. He participated in study concepts, study design, clinical studies and manuscript review. PQL, YGH, WL, LZM, LW, NW, JML, WQC, GML, YMX, YLC and YZ participated in clinical studies, data acquisition and analysis, and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.











