INTRODUCTION
Campylobacter, a Gram-negative zoonotic pathogen, is one of the most widespread infectious agents in the world [Reference Kaakoush1]. It is not only the leading cause of gastroenteritis in humans, but it can also cause serious long-term sequelae like Guillain–Barré syndrome, reactive arthritis, and inflammatory bowel disease [Reference Keithlin2]. The annual incidence of campylobacteriosis varies between countries, but the numbers of reported cases have been increasing worldwide in the past decade [Reference Kaakoush1]. Importantly, estimated burden of disease is considerable, as it is estimated to cost US$1.7 billion/year in the United States alone [Reference Batz, Hoffmann and Morris3].
Chicken consumption and handling have been identified as major risk factors for campylobacteriosis [Reference Harris, Weiss and Nolan4] as broiler chickens are frequently colonized with C. jejuni. Recent studies have also reported attributable rates for cattle in up to 19·3% of Campylobacter cases [Reference Boysen5, Reference Kittl6], suggesting that cattle serve as another important source for human infections. Indeed, raw milk and cheese have been implicated in several outbreaks [7]. Campylobacter is also widespread in the environment including water and soil, where it has been found to survive for several months [Reference Pitkänen8, Reference Jones9]. Water, especially, has been identified as an important source for Campylobacter infections and has been linked to outbreaks as well [Reference Taylor10, Reference Clark11]. Human-to-human transmission via the faecal–oral route has been reported; however, zoonotic or foodborne transmission is the predominant mode. With the high prevalence of campylobacteriosis reported throughout the world, foreign travel has also emerged as an important risk factor [Reference Kendall12].
Seasonal variation has been described for Campylobacter infections. A significantly higher incidence of campylobacteriosis has been reported in warmer seasons in different countries and from different sources such as animals and water [Reference Rind and Pearce13, Reference Kovats14]. The reason behind this seasonality is not known, but has been suggested to be the result of multiple factors including longer survival of Campylobacter sp. in the environment, increased shedding levels in animal reservoirs [Reference Jore15], and changes in human behaviour [Reference Carrique-Mas16]. Spatial determinants such as urban vs. rural settings, have also been linked to campylobacteriosis incidence [Reference Spencer17, Reference Green, Krause and Wylie18], suggesting the need to assess environmental factors when conducting risk-factor analyses.
In the United States, the Foodborne Diseases Active Surveillance Network (FoodNet), which monitors incidence trends of foodborne pathogens, reported an increase in the incidence of campylobacteriosis, from 12·3 cases/100 000 in 2009 to 14·2 cases/100 000 in 2012 [19]. This increasing trend may be partly due to improvements in detection methods and enhanced surveillance, although other risk factors such as geographical location are also likely to be important. Considerable variation in campylobacteriosis incidence was observed among FoodNet sites throughout the surveillance period, ranging from 7 cases/100 000 in Tennessee to 34·3 cases/100 000 in California [19]. Because no significant differences were identified for key risk factors, medical care-seeking behaviour, or medical practices between sites, it is probable that site-specific risk factors like climate or number of animal reservoirs are also contributing to the variable rates [Reference Ailes20].
Because Michigan is not a FoodNet site, we sought to calculate the incidence of campylobacteriosis over a 10-year period using data collected via the Michigan Department of Health and Human Services (MDHHS) and identify risk factors associated with disease. We hypothesized that the incidence of Campylobacter infections has increased temporally and specific factors are associated with increasing incidence. We also investigated clinical outcomes and demographics to assess the disease burden of campylobacteriosis in Michigan and identify characteristics associated with increased risk of infection. Ongoing surveillance efforts are vital to monitor incidence trends and investigate risk factors for disease in different geographical locations in order to more effectively define preventive measures.
METHODS
Campylobacteriosis is a reportable disease in Michigan. A total of 7182 laboratory-confirmed cases were reported to MDHHS with an onset date between 1 January 2004 and 31 December 2013. Demographic, clinical, and epidemiological data for each case were extracted from the Michigan Disease Surveillance System (MDSS) managed by the MDHHS. Season was categorized based on the onset date and travel was considered only when the travel period was within 1 week prior to the onset of symptoms. Data involving history of food consumption and animal contact were systematically collected from 2011 and thus, only the last 3 years of data were used in the analysis. The water source at home was categorized into well, municipal, bottled and other, which included various combinations of the different sources. Data was considered missing for all variables lacking data or that were reported as ‘unknown’.
Age-adjusted incidence rates (cases/100 000) were calculated using the Bridged-Race Population Estimates 1990–2013 dataset [21] and U.S. 2010 standard population by the U.S. Census Bureau [Reference Howden and Meyer22]. Statistical analyses were performed using SAS v. 9·3 (SAS Institute Inc., USA). Differences in the frequencies of campylobacteriosis across variables were examined using χ 2 tests; P < 0·05 was considered significant. Additional analyses were conducted to investigate the association between demographic characteristics and foreign travel history, where the prevalence ratio was compared between individuals with and without a travel history. Multivariate analyses for hospitalization and rural vs. urban residence were performed using logistic regression while adjusting for independent variables with a P value of <0·2 and biologically plausible variables that could represent confounders (e.g. age, sex). The multivariate model was constructed using a forward stepwise method with the requirement for a significance level of P⩽0·1 to remain in the model.
A Geographical Information System (GIS) map was generated in ArcGIS v. 10·2·2 (ESRI, USA) using data from the National Center for Health Statistics (NCHS), the bridged-race population estimates, and the case numbers in this study. Based on the NCHS classification system [23], ten Michigan counties with large metropolitan areas were classified as urban, while the remaining 73 counties were defined as rural. All protocols were approved by the Institutional Review Boards at Michigan State University (IRB no. 10-736SM) and MDHHS (842-PHALAB).
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
The average age-adjusted annual incidence was 7·22 (cases)/100 000, ranging from 6·26 in 2004 to 8·35 in 2013. An increasing trend was observed over time with average incidence rates increasing from 6·58/100 000 in 2004–2006 to 7·37/100 000 in 2007–2010 and 7·65/100 000 in 2011–2013. The average annual incidence was higher for men (7·89/100 000) than women (6·56/100 000), with an incidence rate ratio of 1·2 (P < 0·01). The highest incidence rate was reported in children aged <5 years (14·86/100 000) compared to other age groups (6·76/100 000, P < 0·01). When stratified by sex, boys aged <1 year had the highest incidence (20·5/100 000), although an overall increasing trend in incidence was observed after 5–19 years, particularly for cases aged between 20–29 and >50 years (Fig. 1).

Fig. 1. Average annual sex-specific campylobacteriosis incidence rate by age group and the trend of age-specific incidence rates by year, 2004–2013.
Caucasians comprised 85% of the total cases in Michigan and Campylobacter incidence was significantly higher (6·51/100 000) in this group compared to Asians (4·47/100 000) and African Americans (1·56/100 000) (P < 0·05). Cases self-reporting as Asian were more likely to have a history of foreign travel [prevalence ratio (PR) 4·3], while the opposite was observed in individuals self-reporting as African American (PR 0·7) (Table 1). Travel destinations were also correlated with race, as 52·4% of Asian travellers visited Asia and 47·1% of African American travellers visited Africa. By age, individuals between 20 and 39 years were more likely to develop a Campylobacter infection following foreign travel (PR 1·6), while the opposite was observed in children aged <5 years (PR 0·3) and in the elderly (aged ⩾80 years) (PR 0·1) (Table 1).
Table 1. Demographic characteristics of Campylobacter cases in Michigan (2004–2013) by foreign-travel status

* Only the cases with travel information (n = 6625) were included.
† Sex was unknown in seven cases.
‡ Age was unknown in two cases.
Different seasonal trends were observed for domestic cases (n = 5795) compared to cases with a recent history of foreign travel (n = 830). Domestic cases were more common in the summer months of June and July [odds ratio (OR) 1·6, 95% confidence interval (CI) 1·3–1·9], while cases with foreign travel history were more common in January and February (OR 1·8, 95% CI 1·3–2·4). For domestic cases, the seasonality in summer (June–August) was more prominent for individuals aged 10–59 years, especially compared to the <1 year and ⩾80 years (OR 1·3, 95% CI 1·1–1·6) age groups (Fig. 2).

Fig. 2. Seasonality of Campylobacter cases reported in Michigan by age group, 2004–2013.
Geographical variation in the age-adjusted incidence was observed in the 83 Michigan counties (Fig. 3). Using data from the NCHS system, ten of the counties were defined as urban and the remainder were rural. The incidence was significantly higher in rural (7·78/100 000) compared to urban areas (6·70/100 000, P < 0·05). Furthermore, the incidence increased by 41·5% from 6·45/100 000 in 2004 to 9·27/100 000 in 2013 in rural cases, relative to a 26·2% increase among urban cases (6·13/100 000 in 2004 to 7·65/100 000 in 2013).

Fig. 3. Age-adjusted incidence of Campylobacter reported in Michigan by county, 2004–2013. Based on the classification by the National Center for Health Statistics (NCHS) data system [23], ten counties, which represent large metropolitan areas, were classified as urban (red outlines) and the remaining 73 counties were classified as rural. The black circles represent the population size of each county.
To investigate the possibility that geographical variation is linked to factors specific to rural areas, we conducted a case-case analysis between the urban and rural domestic cases. The age-specific incidence was higher for rural cases compared to urban cases, especially for groups aged 10–19 years [incidence rate ratio (IRR) 1·6], 20–39 years (IRR 1·3), and >80 years (IRR 1·3). Univariate analyses demonstrated that contact with animals (i.e. ruminants, poultry, domestic pets) was significantly more common for rural cases compared to urban cases as well as consumption of raw milk, ground meat, and frozen chicken (Table 2). The most notable difference, however, was the water source at home as rural cases were significantly more likely to drink only well water (OR 7·3, 95% CI 5·6–9·4). Multivariate logistic regression controlling for age and sex identified contact with ruminants (OR 1·6, 95% CI 1·0–2·5), consumption of ground meat (OR 1·4, 95% CI 1·1–1·7), and well water at home (OR 5·4, 95% CI 3·9–7·4) to be independently associated with Campylobacter infection in rural areas.
Table 2. Univariate and multivariate analyses of risk factors in rural cases (n = 1035) compared to urban cases (n = 880)
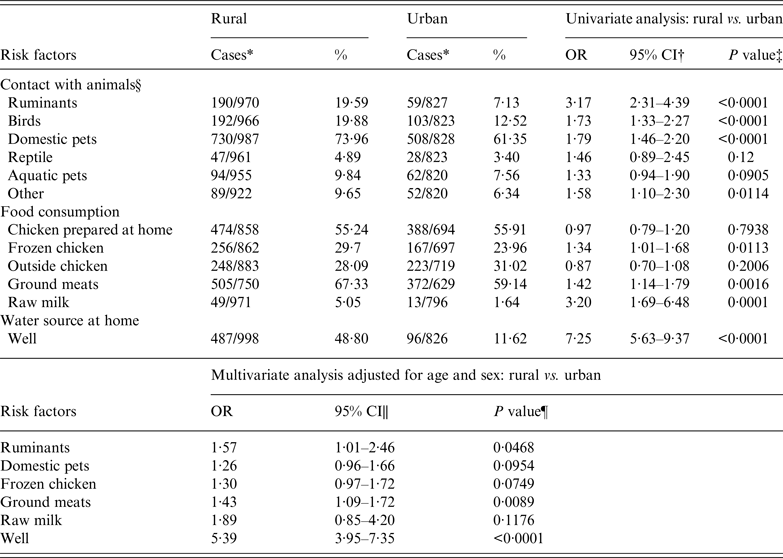
OR, Odds ratio; CI, confidence interval.
* Only the cases with onset date between 2011and 2013 (n = 1915) are included due to the high proportion of missing data in previous years.
† 95% confidence interval for odds ratio.
‡ Likelihood ratio χ 2 test.
§ Animal contact was defined as positive when there was a report of direct contact with reptiles (e.g. snake, lizards), ruminants (e.g. cattle, goats, sheep), birds (e.g. chickens, turkeys, ducks, parrots), aquatic pets (e.g. fish, turtles), domestic pets (e.g. dogs, cats) and other animals. High-risk food exposure was evaluated by consumption history of ground meat (e.g. turkey, chicken, beef, pork), chicken, (i.e. prepared at home, frozen, or at a restaurant), and unpasteurized milk or cheese within a week. Frozen chicken was defined as chicken items that are sold frozen (i.e. breaded, pre-browned, stuffed). The water source at home was categorized into well, municipal, bottled and other, which included combinations of different sources.
|| Wald confidence interval.
¶ Wald χ 2 test.
Given the association with campylobacteriosis and ruminants, we evaluated the ecological association between animal density (i.e. cattle, chickens, goats, sheep, pigs) and disease incidence by county using data from the National Agricultural Statistics Service for 2012 [24]. Counties with <1% of the total number of each animal species in the state were classified as low-density counties, while counties with ⩾1% were classified as high density. Notably, 12 counties with the highest incidence rates of campylobacteriosis (13·6–35·1/100 000), all of which were also defined as rural areas (Fig. 3), were more likely to have a high cattle density (OR 2·5, 95% CI 2·1–2·9). No significant associations were observed for other animal species including chicken.
Clinical outcomes also varied in cases over time; the hospitalization rate increased from 23·3% in 2004 to 29·5% in 2013. Cases aged ⩾60 years had a significantly higher likelihood of hospitalization than other age groups (OR 2·5, 95% CI 2·2–2·8), while bloody diarrhoea was more common in children aged <5 years (OR 2·5, 95% CI 2·1–2·9). Based on these prior associations, we also sought to determine whether there were differences in hospitalization status among cases in rural vs. urban areas and after stratifying by travel history. Both urban and domestic cases were more frequently hospitalized compared to rural cases and those with foreign travel history (Table 3). Multivariate analyses demonstrated that age ⩾60 years (OR 2·4, 95% CI 2·1–2·7), lack of foreign travel (OR 2·6, 95% CI 2·1–3·2), and urban residence (OR 1·2, 95% CI 1·1–1·4), were independently associated with hospitalization.
Table 3. Univariate and multivariate analyses of characteristics associated with hospitalization due to campylobacteriosis
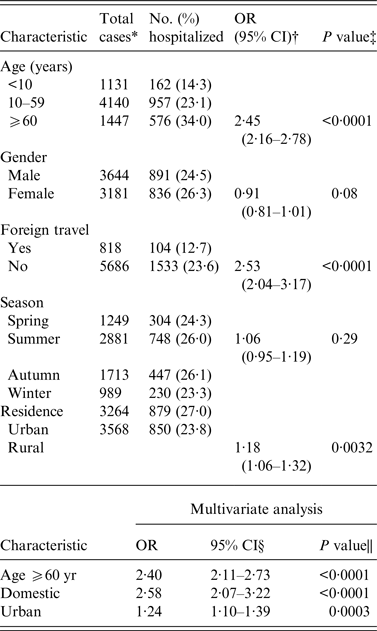
OR, Odds ratio; CI, confidence interval.
* Total number differs between variables due to missing information.
† 95% confidence interval for odds ratio.
‡ Likelihood ratio χ 2 test.
§ Wald confidence interval.
|| Multivariate logistic regression.
DISCUSSION
This study represents the first temporal report of campylobacteriosis incidence in Michigan and highlights an increasing incidence trend in the last decade. This increase may be partly due to enhanced awareness and changes in detection practices [Reference Hurd25], although other factors are also likely to be important. When stratifying incidence by age, for example, different trends were observed. Increased incidence in cases aged between 20–29 and >50 years suggests that age-specific risk factors such as diet or other lifestyle factors, may be important for disease. Indeed, a higher attributable risk was observed for foreign travel in individuals aged between 20 and 39 years compared to other age groups, while higher disease frequencies occurred in the summer months for those aged between 10 and 59 years, especially compared to the <1 and ⩾80 years age groups. This finding suggests that seasonality could be impacted by summer-related behaviours including camping, swimming, or outdoor grilling, but also that these behaviors may be important risk factors for individuals aged between 10 and 59 years. Additional epidemiological studies are warranted, however, to fully investigate age-specific risk factors for the design of novel public health interventions.
Other factors associated with Campylobacter seasonality may include humidity and temperature [Reference White26] as high humidity and temperature levels enhance pathogen survival and proliferation, potentially increasing the load in animal reservoirs [Reference Jones9, Reference Jore15]. Insect vectors like flies can also facilitate transmission of Campylobacter sp. between animal reservoirs, as well as to humans, when there is increased ventilation and airflow during months with warmer weather [Reference Hald27, Reference Nichols28]. In our study, only domestic cases showed a marked increase in incidence in the summer months, supporting the idea that factors important for seasonality are linked to geographical location or specific environments.
A significant difference in Campylobacter incidence was observed by geographical location, particularly when counties were classified as urban or rural. The higher incidence observed in rural cases in this study is most likely due to more frequent contact with animals and environmental exposures such as well water. Similar findings have been reported previously, as direct contact with farm animals [Reference Kaboré29, Reference Gilpin30], swimming in lakes and rivers [Reference Dale31], and drinking untreated water [Reference Karagiannis32], were associated with a higher risk of campylobacteriosis in rural settings. The steep increasing rate observed in rural areas of Michigan is concerning and warrants a further investigation to identify the source of infection in these areas.
Although chickens are a major reservoir for Campylobacter sp. and a prior Michigan study noted a correlation between poultry density and campylobacteriosis incidence [Reference Potter, Kaneene and Gardiner33], poultry density was not significantly associated with incidence in our study. Indeed, only four of the 83 counties were classified as having high chicken densities and one of these counties comprised 96·3% of the total layers in Michigan. Broilers were the dominant poultry type in the other three counties. The fact that poultry farming was confined to only four Michigan counties may have limited our ability to detect an association between poultry density and campylobacteriosis.
By contrast, 30 counties were classified as high cattle counties, in which the number of dairy cows was 3·6 times higher than beef cows. Compared to another prior Michigan study conducted in 2003 [Reference Potter, Kaneene and Hall34] that reported poultry husbandry as the key risk factor for campylobacteriosis in rural residents, only ruminant contact was a risk factor in rural cases compared to urban cases in our study. Although cattle contact would be expected to be more common and frequent in rural residents, it is plausible that the elevated risk for rural cases with well water may be suggestive of faecal contamination of source waters. Of rural cases, those in counties with high cattle densities were more likely to have well water as the only water source compared to cases from low-cattle-density counties (OR 1·6, 95% CI 1·2–2·1). Nonetheless, further studies are warranted to elucidate the genetic relatedness of isolates recovered from humans and cattle as well as the environment, including private wells, to better address questions involving source attribution and transmission dynamics. Additional studies are also needed to better assess associations between disease and food handling, preparation and consumption practices. Even though we initially observed an association between disease and frozen chicken consumption in the univariate analysis for rural cases, this association was no longer significant in the multivariate analysis.
Population distribution by race may also affect the overall incidence of Campylobacter in a given area. In this study, Caucasians had significantly higher rates relative to individuals of other races, especially African Americans, although different patterns were observed when foreign travel status was compared by race. These data suggest that risk factors may vary by race, which is similar to a prior study that reported a different level of risk for travel-related Campylobacter infections by the travel destination [Reference Mughini-Gras35]. More specifically, they observed higher levels of infection following travel to Southeast Asia (32·4%), South Asia (7·8%), Africa (4·6%), and Latin America (2·5%). Another study also observed an association between a higher socioeconomic status and a higher campylobacteriosis notification rate [Reference Bemis, Marcus and Hadler36], thereby warranting future studies aimed at examining the association between racial/ethnic background and disease while taking socioeconomic status into account as a confounding factor.
Similar to studies conducted in Hungary [Reference Sonnevend37] and the UK [Reference Gillespie38], we observed a significant association between age and severe symptoms as children aged <5 years more frequently reported bloody diarrhoea. Since these findings are consistent across geographical locations with distinct strains in circulation, we expect that an immature intestinal mucosal immune system may contribute to more severe complications in young children [Reference Gillespie38, Reference Davies, AGM and N39] and this is not the result of ascertainment bias. Although the association with hospitalization identified in the study is important, it may represent a ‘healthy travellers effect’ [Reference Ternhag40] as limited access to medical facilities may have contributed to lower hospitalization rates in travellers and rural cases. Nonetheless, the increasing rate of hospitalization in individuals aged ⩾60 years is concerning and may be due to the increasing Campylobacter incidence in this population.
In summary, the incidence of campylobacteriosis has increased in Michigan over time, and considerable variation was observed in the spatial distribution of cases and in factors associated with infection. Continuous monitoring of incidence is warranted, while additional studies must attempt to identify those individuals who are most susceptible to campylobacteriosis and more severe clinical outcomes. Designing target-specific preventive measures for the most susceptible population and identifying major environmental sources may be a key to decreasing infection rates and the disease burden in specific geographic locations.
ACKNOWLEDGEMENTS
This study was funded by the National Institutes of Health Enterics Research Investigational Network (ERIN) Cooperative Research Center at Michigan State University (S.D.M., grant number AI090872) and the United States Department of Agriculture, National Institute of Food and Agriculture (S.D.M., grant no. 2011-67005-30004).
DECLARATION OF INTEREST
None.









