Rubella is generally a mild infectious disease. However, during pregnancy it may cause serious congenital malformations in the foetus known as congenital rubella syndrome (CRS). Rubella and CRS can be prevented by vaccination [Reference Banatvala and Brown1] and in The Netherlands rubella vaccination was introduced for girls only as part of a national vaccination programme in 1974. In 1987, this was replaced by the two-dose measles-mumps-rubella (MMR) vaccination for all children. Despite vaccination coverage of >95%, a rubella epidemic occurred in 2004/2005 and similar to rubella outbreaks among the Amish in the USA [Reference Briss2], this epidemic was largely confined to an orthodox Protestant minority group that refrains from vaccination for religious reasons [Reference Hahné3].
To prevent CRS, all young women should be protected against rubella either via vaccination or the acquisition of a natural immunity prior to childbearing age. During the 2004/2005 epidemic, the Dutch municipal health services offered free MMR vaccination to unvaccinated children and adolescents. However, the acceptance of this was very limited. Similarly, a personal recall for missed vaccinations to unvaccinated 16-year-olds (as registered in the Provincial Vaccination Register) also showed only 7% vaccination acceptance [Reference Aardoom4].
In contrast to the low vaccination acceptance rates, the unvaccinated rubella patients and their parents proved very willing to undergo diagnostic procedures to confirm rubella infection during the epidemic despite the results having no therapeutic consequences for them. Given the interest of unvaccinated girls and their parents in the serostatus of the girls, it was decided to develop a screening programme to detect rubella susceptibility among unvaccinated young women, offer MMR vaccination to those found to be seronegative, and thereby increase protection against rubella. This strategy has been suggested by others [Reference Robinson5], and the objective of the present study was therefore to test the feasibility of such a strategy.
All 640 women aged 14–20 years from two villages with large unvaccinated orthodox Protestant populations were invited to take part in the study. Overall MMR vaccination coverage in these villages was 63% for the birth cohorts invited for the study. The target group of the programme was unvaccinated young women but to avoid stigmatization, the serological test was offered to all young women, irrespective of vaccination status.
Invitations for the serological test and questionnaires accompanied by an informed consent form were mailed to all of the women in the study population. For girls aged <18 years, the parents were approached and asked to provide written consent.
Vaccination status was assessed retrospectively via the questionnaire. Women who did not know their vaccination status were assumed to be unvaccinated.
Blood samples were taken in the villages by nurses from the municipal health service.To foster participation, the blood samples were collected via finger prick, which has been shown to allow sufficiently sensitive serological testing relative to testing of serum collected via venepuncture [Reference Helfand6, Reference Condorelli7]. Blood obtained via finger prick was spotted on filter paper and dried. The blood specimen was reconstituted in the laboratory and tested for the presence of rubella-specific IgG antibodies (Enzygnost®, Dade Behring GmbH, Germany). Rubella IgG test results <4 IU/ml were classified as negative; test results ⩾15 IU/ml were classified as protective; test results between 4 and 14 IU/ml were classified as equivocal. Women with initially equivocal test results were asked to provide a second blood sample but now via venous puncture. These blood samples were again tested for rubella IgG using another test (AxSYM®, Abbott Diagnostics, USA) due to the different logistics associated with the collection of the different blood samples. Venous blood test results ⩾15 IU/ml were considered protective.
All of the participants received personal written feedback regarding the laboratory results. Unprotected women were offered MMR vaccination free of charge.
Only the analyses of the data from the subgroup of previously unvaccinated participants are presented here. Discussion of waning immunity among the subgroup of vaccinated women is beyond the scope of this report. The data were analysed using SPSS software, version 13 (SPSS Inc. USA). Percentages were calculated for participation, rubella susceptibility, and acceptance of vaccination. The different subgroups of participants classified according to religious denomination, age, and education were compared using Fisher's exact tests.
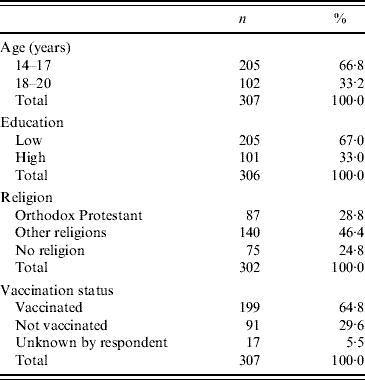
The participation in our study was 48% (95% CI 44–52). A total of 307 women participated in the study of whom 108 (35%, 95% CI 30–41) belonged to the target group of unvaccinated women. The characteristics of the participants are presented in Table 1. Vaccination status was significantly related to religious denomination. The majority of the unvaccinated women (77 women, 71%) belonged to orthodox Protestant denominations. Vaccination status was not associated with age or educational level.
Table 1. Characteristics of respondents
Eleven per cent (95% CI 6–19) of the unvaccinated women were susceptible to rubella. The results of the serological testing of the finger prick blood from 108 unvaccinated women showed five (5%) to be seronegative, 10 (9%) to have equivocal results and 93 (86%) to be protected against rubella. After venous blood testing for those with initially equivocal results, three more women could be considered protected as their venous rubella IgG was ⩾15 IU/l; five women were unprotected, and two women did not return for repeated testing and were therefore also considered unprotected. Therefore, 12 (11%, 95% CI 6–19) unvaccinated women were considered susceptible to rubella. Rubella susceptibility in the unvaccinated women was clearly associated with religious denomination. A higher percentage of the women belonging to orthodox Protestant religious denominations were protected against rubella when compared to the women belonging to other religious denominations or women with no religious denomination (Table 2). In addition, a higher percentage of the younger unvaccinated women (i.e. those aged 14–17 years) were protected against rubella when compared to the older group (i.e. those aged 18–20 years). Rubella susceptibility in unvaccinated women was not associated with educational level.
Table 2. Rubella susceptibility of unvaccinated women according to religion and age (n=108)
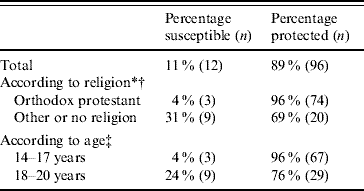
* Information on religious denomination was missing for two respondents.
† Fisher's exact test: P<0·001.
‡ Fisher's exact test: P=0·004.
Only 17% (2/12, 95% CI 2–48) of rubella-susceptible, formerly unvaccinated women agreed to subsequent MMR vaccination by the municipal health service. Both women did not belong to an orthodox Protestant denomination.
These outcomes were used to assess the feasibility of the screening and vaccination programme. The efficiency of the programme is dependent on the participation rate of the target group, their rubella susceptibility and the acceptance of vaccination by those susceptible. Thus it can be concluded that 0·48×0·11×0·17×100=0·9% (95% CI 0·1–2·5) of the target group of unvaccinated young women was provided protection by the programme. In other words: the invitation to 100 unvaccinated young women for rubella screening will lead to acceptance of a vaccination offer by only 1 susceptible woman. Ten women will remain susceptible because they do not agree to screening (0·52×0·11×100=6 women) or refuse vaccination after testing seronegative (0·48×0·11×0·83×100=4 women). Eighty-nine out of 100 unvaccinated women are already protected by naturally acquired immunity.
Our results show that rubella screening of unvaccinated women prior to childbearing age, followed by the offer of MMR vaccination to those who tested seronegative has only a very limited effect on rubella protection in an area with low vaccination coverage due to religious objections.
The participation rate in our study was 48%. As the vaccination coverage found for our study population is consistent with the historical vaccination coverage, it could be assumed that the participation rate for the target group of unvaccinated young women was independent of their vaccination status and equal to the overall participation rate. Moreover, the participation rate found in the present study is comparable to the participation rate of 52·5% found in a 1996 population-based immunosurvey of low vaccine coverage municipalities in The Netherlands [Reference de Melker and Conyn-van Spaendonck8]. The necessity of parental consent for girls aged <18 years did not affect participation. The participation rate for the younger group was even higher than the participation rate for the older group. The aim of the screening and vaccination programme is to prevent rubella in pregnancy; therefore, the fact that for most women aged between 14 and 20 years, pregnancy is not yet an issue may contribute to the relatively low participation rates we found. Preconception rubella screening and vaccination may thus result in higher participation rates and has recently been recommended by the Dutch Health Council [9].
Rubella susceptibility among unvaccinated young women was found to be only 11%, which reflects the high likelihood of naturually acquiring the rubella infection in these villages. Before the 2004/2005 epidemic there must also have been circulation of rubella virus in The Netherlands, as rubella seroprevalence in areas with low vaccination coverage in the 1996 population-based serosurvey for the same generation of unvaccinated youth – who were then aged 5–10 years – was already about 65% [Reference Haas de10]. The higher seroprevalence in unvaccinated orthodox Protestant women relative to other groups of unvaccinated women is consistent with the observation that most cases in the 2004/2005 epidemic were found to occur in groups who refrained from vaccination for religious reasons [Reference Hahné3]. Similarly, the finding that the 14- to 17-year-old unvaccinated women were better protected against rubella than the 18- to 20-year-old women may reflect the fact that the younger age group had a higher probability of exposure during attendance at secondary school (often an orthodox Protestant school) than the older age group, which no longer attended school.
The acceptance of the vaccination offer by the previously unvaccinated seronegative women was limited. However, we were unable to assess the acceptance of the offer with much precision due to the small number of women identified as susceptible. Nevertheless, the reasons for low vaccination acceptance may be similar to the reasons mentioned for low participation. Religious objections may also give rise to a conflict of conscience on the part of unprotected young women in particular. Moreover, as pregnancy is not as yet an issue, the decision to accept vaccination can also be postponed. Therefore, it is possible that a vaccination offer following preconceptional screening will result in higher rates of vaccination acceptance.
Applying the Wilson–Jungner criteria for mass screening adopted by the WHO in 1968, we conclude that there is a serious health problem, a suitable test and an appropriate ‘treatment’ (i.e. vaccination). However, at the observed levels of participation, rubella susceptibility and vaccination acceptance, the screening of unvaccinated women prior to child bearing age is most probably not a cost-effective strategy in The Netherlands and therefore not recommended.
ACKNOWLEDGEMENTS
We thank Dr A. M. van Loon, University Medical Center Utrecht for rubella IgG testing on finger prick blood specimens. This study was financially supported by Fonds OGZ, a fund established by the Dutch government to stimulate public health research.
DECLARATION OF INTEREST
None.




