Introduction
Rotavirus diarrhoeal disease has a significant impact on childhood morbidity and mortality, as well as on health costs. The World Health Organization estimated that in the pre-vaccination era, rotavirus caused more than 450 000 deaths annually in children under 5 years old worldwide in 2008, most of them in developing countries [Reference Tate1]. More recently, 2013 estimates accounted for 215 000 deaths in that age group [Reference Tate2]. However, rotavirus remains the leading cause of diarrhoea cases, hospital admissions and deaths [Reference Operario3]. In Argentina, in the absence of a massive vaccination strategy, rotavirus has been estimated to cause 150 000 acute diarrhoea cases, 15 000 hospital admissions and around 30 deaths each year in children under 5 years of age [Reference Degiuseppe4]. This virus has also been responsible for about 25% of all-cause acute diarrhoea cases in this age group, with a seasonal peak of activity in the cold months [Reference Degiuseppe4, Reference Degiuseppe5].
Since vaccine approval and licensing in 2006, the rotavirus massive vaccination strategy has been considered the most effective public health measure for reducing this disease burden [Reference Karafillakis, Hassounah and Atchison6–Reference Velázquez8]. Therefore, the World Health Organization has been continuously encouraging countries to include it in their National Immunization Programmes [9]. Currently, two oral rotavirus vaccines, a three-dose, pentavalent human-bovine reassortant vaccine (RotaTeq® Merck) and a 2-dose, monovalent, attenuated human rotavirus vaccine (Rotarix® GSK), are licensed for use [Reference Ruiz-Palacios10, Reference Vesikari11]. Although Latin America has seen a rapid and successful introduction of rotavirus vaccines, Argentina only incorporated Rotarix into its National Immunization Programme since 1 January 2015 [Reference Velázquez8, Reference de Oliveira12]. After two complete years of introduction, the reported coverage of one-dose and two-dose rotavirus vaccine in eligible Argentine children had reached around 85% and 75% nationwide, respectively (Devoto, personal communication). In Argentina, all-cause acute diarrhoea cases are included in the Mandatory Notification Events list. The Argentine Health Surveillance System (SNVS; www.snvs.msal.gov.ar), which depends on the National Ministry of Health, is an online software tool comprising two main modules: clinical (C2) and laboratory-based (SIVILA) surveillance. Particularly with diarrhoeal diseases and rotavirus cases, official public and private hospitals distributed nationwide register weekly the total number of cases medically attended, disaggregated into age groups. Unfortunately, no specific strategy has yet been designed to accurately measure the impact of this recent introduction of rotavirus vaccine on the diarrhoeal disease burden in our country.
Another concern is that vaccine introduction has been reported to produce rapid changes in the rotavirus circulating genotype patterns. More specifically, in countries that had implemented the monovalent vaccine, an increase in the detection of G2P[4] association was observed in residual cases of rotavirus diarrhoea, and an increase of G3P[8] when the pentavalent vaccine was used as massive strategy [Reference Kirkwood13–Reference Zeller16]. These findings underscore the importance of local monitoring of circulating rotavirus strains before and after vaccine introduction.
Consequently, the aim of this study is to assess post-vaccine introduction data (all-cause acute diarrhoea and laboratory-confirmed rotavirus cases, and genotype distribution) compared with the pre-vaccination period in children under 5 years of age in Argentina.
Methods
Study design
This study was an observational, cross-sectional, ecologic analysis of all-cause acute diarrhoea and laboratory-confirmed rotavirus cases in children under 5 years of age, reported to the SNVS in Argentina in 2016. It included 23 of the 24 Argentine provinces. San Luis Province was excluded because it implemented rotavirus massive vaccination strategy in mid-2013 [17]. However, children under 5 years from San Luis represented only 1.1% of the total 2016 national estimated population in this age group [18].
All-cause acute diarrhoea cases
Data on all-cause acute diarrhoea cases in children under 5 years were accessed from the Condensed Clinical Notification Module of the SNVS-C2. This module includes, at provincial level, weekly information on all reported health events seen in official public and private hospitals, disaggregated into age groups (<1, 1–4, 5–9, 10–14, 15–24, 25–34, 35–44, 45–64, >65 years and age unspecified).
Endemic channel
We extracted weekly totals of all-cause acute diarrhoea cases in children under 5 years by province and annual population projections to determine the endemic channel as previously described [Reference Bortman19]. Briefly, weekly cases of a given event from the year of interest (2016) and the 5 previous years (2011–2015) were listed, and the median and quartiles were calculated to assess four different epidemiological zones: success, safety, alert and epidemic. For national analyses, we summed data from 23 Argentine provinces.
Global and seasonal incidence rates
We obtained annual data from the 23 provinces by summing 52 weekly values in the 2011–2016 period. To aid comparison, we calculated the mean of all-cause acute diarrhoea global incidence rates from the 2011–2014 period (pre-vaccination) and from 2016 (post-vaccination). Considering 2015 period a transition year during which rotavirus vaccine was introduced, it was excluded from these analyses. As a denominator, we used annual population projections for each year and for each province from the Argentine Institute of Statistics and Census [18]. Weekly and global data were further disaggregated by sub-age group (<1 and 1–4 years). Also, considering that the highest rotavirus activity has been widely described in Argentina between epidemiological weeks (EWs) 16–36 [Reference Degiuseppe4, Reference Degiuseppe5], weekly data were further grouped into three annual seasons (EW 1–15, EW 16–36 and EW 37–52). To monitor whether diarrhoea incidence rates might vary beyond our group of interest, we designated the 15–64 years old group as control. For comparative purposes, we calculated rate ratios (RR) and 95% confidence intervals (CI) between pre- and post-vaccination periods by using Poisson regression analysis. Statistical analyses were done with the OpenEpi online tool (http://www.openepi.com).
Rotavirus laboratory-confirmed cases
Data were taken from the Viral Diarrhoea Notification Module of the SNVS–SIVILA. Briefly, nationwide hospital laboratories upload a weekly condensed report on the total amount of rotavirus tests performed and the number of positives by either ELISA or immunochromatography, classified by age group. To ensure proper and constant coverage, we used data from laboratories that had been reporting for at least 44 EWs (~85%) per year to the National Reference Laboratory during the studied period. Seasonal peak was defined as the three consecutive week period with the highest number of rotavirus cases. The proportion of positive tests was calculated from positive and negative rotavirus cases. In order to aid analysis, we compared 2016 data for children under 5 years (post-vaccination period) to the 2011–2014 mean (pre-vaccination period). Interannual comparison analysis was assessed by contingency tables, using χ 2 test and considering P-value <0.05 significant.
Rotavirus circulating genotypes
Analyses of circulating genotypes were performed on all the rotavirus-positive stool specimens sent by hospital laboratories to the National Reference Laboratory according to the rotavirus surveillance protocols. Also, conventional binary characterisation of the outermost capsid genes (VP7 and VP4 for G and P typing, respectively) used a hemi-nested multiplex RT-PCR as previously described. Briefly, the entire VP7 gene was first amplified with the Beg9/End9 pair of primers [Reference Gouvea20], and the second amplification was done with the 9Con1, 9T-1, 9T-2, 9T-3, 9T-4 and 9T9B set of primers [Reference Das21]. For VP4 amplification, the Con3/Con2 pair of primers and 1T-1, 2T-1, 3T-1, 4T-1 and 5T-1 set of primers were used for the first and second amplification, respectively [Reference Gentsch22]. The first-round amplicons of untyped G-types were further analysed by nested PCR using different sets of G5, G8 and G12 type-specific primers for the VP7 gene [Reference Gouvea20, Reference Gouvea, Santos and Timenetsky23, Reference Pun24]. To confirm the results of the G/P typing methods involved, we also randomly selected 25% of different strains for VP7 and VP4 gene partial nucleotide sequencing and further confirmation by using the BLAST program [Reference Altschul25]. Also, to obtain additional information on the genetic backbone, we amplified and sequenced the VP6 and NSP4 gene segments of the most prevalent genotypes detected (in two randomly selected samples from each strain) as described previously [Reference Matthijnssens26]. Genotype assignment was done with the RotaC v2.0 online software tool [Reference Maes27].
Results
All-cause acute diarrhoea cases
According to the notifications on the SNVS-C2 module, the endemic channel of all-cause acute gastroenteritis cases of 2016 showed a fluctuation between safety, alert and outbreak zones during the first 9 weeks of the year (the warm season). From EWs 10 to 36, cases showed a significant decrease compared with previous years, and moved into the success zone. Between EWs 37 and 41, cases returned to the safety and alert zone, and at the end of the year, cases were located in the success zone (Fig. 1). The mean global rate of gastroenteritis cases in the pre-vaccination period (2011–2014) was 142.1 per 1000 children under 5 years and in the post-vaccination period (2016) was 112.6 (Table 1). Therefore, a global decrease of 20.8% was found (RR 0.792, 95% CI 0.789–0.795). When subgrouping by age, the under 1-year-old group showed a significant reduction between pre- and post-vaccination period rates (RR 0.684, 95% CI 0.678–0.690), which contrasted with the 1–4 years old group, with a lower decline (RR 0.827, 95% CI 0.823–0.830). Seasonal analyses showed that the highest rate reduction in children under 5 years of age was observed in the EWs 16–36 season (RR 0.575, 95% CI 0.571–0.579), mainly due to a decrease in the under 1 year group (RR 0.441, 95% CI 0.434–0.448). The 15–64 years group also showed a reduction in global and seasonal all-cause acute diarrhoea rates (Table 1). In comparison with the under 5 years group, the decline was lower in the adult group except in the EW 1–15 season. Also, the highest rate reduction was observed in the EW 16–36 season (RR 0.746, 95% CI 0.740–0.751) for this group.
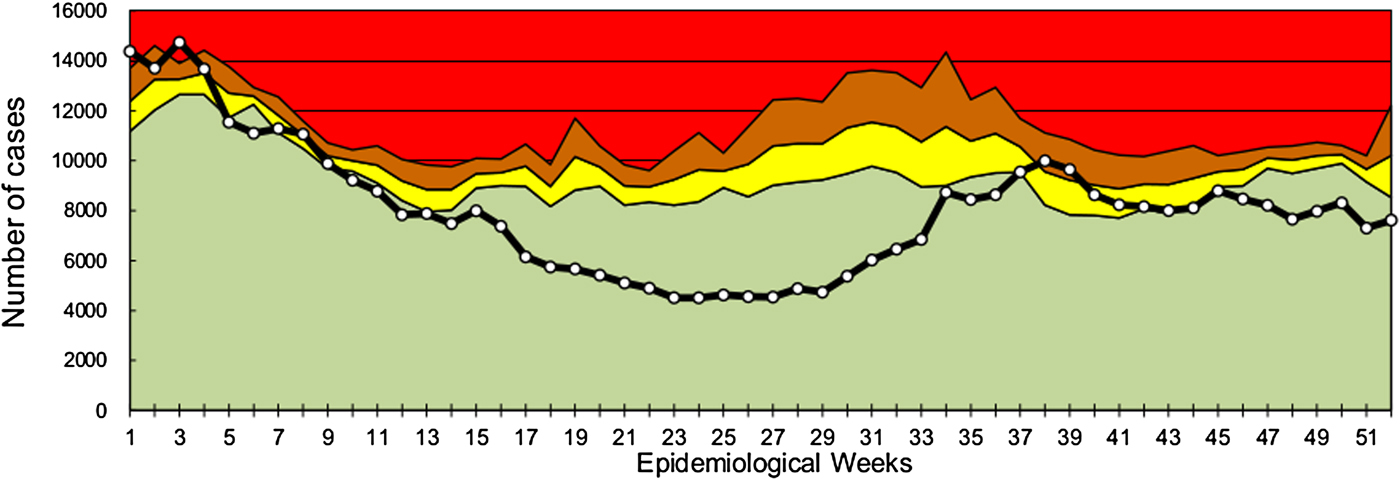
Fig. 1. All-cause acute diarrhoea endemic channel in children under 5 years in Argentina, 2016. White-dotted solid black line indicates weekly all-cause acute diarrhoea cases in children under 5 years. Data from previous 5 years (2011–2015) were used to assess four epidemiological zones (referenced by colours): success (green), safety (yellow), alert (orange) and epidemic (red).
Table 1. All-cause acute diarrhoea rates (per 1000), rate ratio (RR) and 95% confidence intervals (95% CI) by age group and seasons in pre- (2011–2014, mean) and post- (2016) rotavirus vaccination periods

Rotavirus laboratory-confirmed cases
In 2016, 704 laboratory-confirmed rotavirus cases in children under 5 years of age were notified through the SNVS–SIVILA module: a global prevalence of detection of 13.6% (Table 2). This represented a 61.7% reduction of confirmed cases compared with the pre-vaccination mean period (2011–2014; n = 1836). We also found a 24.5% reduction in tests performed. When analysing sub-age groups, children under 1-year old showed a 57.5% reduction in the proportion of positive tests compared with a 41.9% decline in children 1–4 years old (P < 0.001). In the pre-vaccination period, the weekly distribution of confirmed rotavirus cases showed a typical autumn/winter seasonal peak (EW 25–35). However, in 2016, we observed significant flattening of the expected seasonal peak. The highest number of rotavirus cases was concentrated between EWs 33 and 39. This shows that the seasonal peak has slightly shifted to the right by 6–8 weeks (Fig. 2).
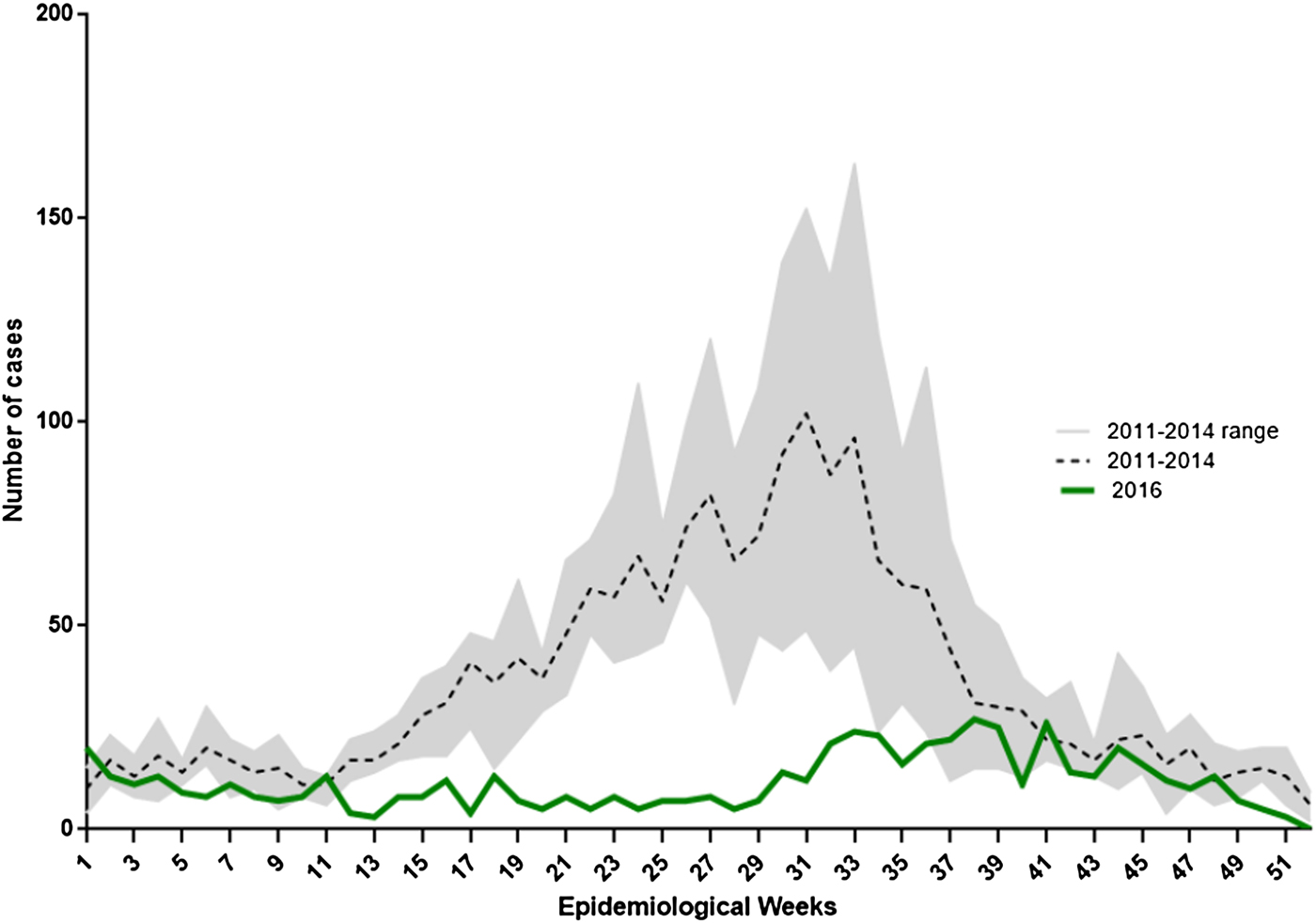
Fig. 2. Weekly distribution of laboratory-confirmed rotavirus cases in children under 5 years of age in Argentina, 2011–2014 and 2016. Lines indicate the number of rotavirus-confirmed cases notified to SNVS–SIVILA according to epidemiological week (EW) for 2016 and 2011–2014 mean (reference code provided).
Table 2. Rotavirus frequency of detection and age group distribution in Argentina in pre- (2011–2014, mean) and post- (2016) vaccination periods

a RFP, relative frequency proportion.
Rotavirus circulating genotypes
Of all positive rotavirus samples received, 208 (~98%) were suitable for rotavirus G- and P-genotypification. G2P[4] was the most prevalent circulating genotype (57.2%), followed by G3P[8] and G9P[8] (15.9% each). Therefore, these three G-/P-associations accounted for nearly 90% of all genotypes detected (Fig. 3). Additional genetic background analyses (VP6 and NSP4 genotypification) showed that the three most frequently detected genotypes bore the G2P[4]-I2-E2, G3P[8]-I2-E2 and G9P[8]-I1-E1 constellation, respectively.

Fig. 3. Rotavirus circulating genotype distribution in Argentina, 2011–2014 and 2016. Proportion of different rotavirus genotypes circulating in Argentina in the 2011–2016 period is described according to the reference code. The horizontal coordinate indicates the amount of G/P typed samples per year.
Discussion
This study represents the first assessment of diarrhoeal disease burden since rotavirus massive vaccination strategy was implemented in Argentina.
The post-vaccination period has shown a significant decrease in all-cause acute diarrhoea cases in children under 5 years compared with the pre-vaccination period (20.8%), and weekly analysis revealed that this decline was concentrated in the autumn/winter season (42.5%). The largest reduction was observed in the under 1-year group (55.9%). Importantly, the 1–4 years group also showed a significant decline in rates after vaccination strategy was implemented (17.3%), which differs from reports from other countries where the effect in the early years was not seen so clearly in the latter age group [Reference Dulgheroff28, Reference Desai29]. Previous studies had indicated that rotavirus in Argentina is responsible for about 25% and 35% of acute diarrhoea cases in children under 5 years and under 1 year, respectively. Therefore, the reductions we observed are similar or even slightly superior to the estimated rotavirus burden. Far from expecting that rotavirus acute diarrhoea cases have been completely reduced, we hypothesise that massive rotavirus vaccination strategy has demonstrated a positive impact not only on specific cases but also on all-cause acute diarrhoea cases in Argentina, as described in many countries [Reference Richardson30–Reference Santos32]. Noteworthy, diarrhoea cases have also been reduced in the adult group in the post-vaccination period. We cannot accurately explain this finding considering that no particular public health intervention was implemented. However, the decrease in the under 5 years group exceeded it by 10% globally and by around 23% in the autumn/winter season. These results highlight the shifting of diarrhoeal disease burden, particularly in children, and the indirect benefits (i.e. herd immunity) for other groups [Reference Lopman33, Reference Mast34].
Laboratory-confirmed rotavirus cases were also successfully reduced in the post-vaccination period (around 60%). Notably, the typical seasonal peak was blunted, and most confirmed cases were reported around 6–8 weeks later than expected. These results concord with others described elsewhere [Reference Zeller16, Reference Tate35, Reference Luchs36].
In contrast with the upward trend observed for G1P[8] and G12P[8] in the pre-vaccination period, rotavirus G2P[4] genotype association was the most predominant nationwide in 2016. This phenomenon has already been reported in many countries that had implemented the monovalent vaccine [Reference Kirkwood13, Reference Gurgel15, Reference Zeller16, Reference Luchs36]. It would seem that massive vaccination leads to a selective contraction and homogenisation of circulating genotypes and, considering that G2P[4] is the antigenically most distant association, it prevails among residual cases. This hypothesis is supported in our study by the detection of G3P[8] strains with DS-1-like backbone. We have found that around 75% of the genotypes detected in symptomatic children bore a genotype constellation genetically distant from the vaccine strain. On the other hand, constant monitoring of local genotype distribution is needed to assess whether these findings represent a permanent switch or result from natural fluctuation dynamics.
This study has some limitations. First, even though all-cause acute diarrhoea cases are included in the Argentine Mandatory Notification of Events, under-notification is expected because this self-limited clinical condition prevents detection of all the cases, and also, institutional commitment to national health information systems may vary. Also, rotavirus laboratory testing is subject to the real demand of local physicians. Therefore, it is important to note that all the analyses of this study were carried out under the assumption that under-notification is random and unbiased. Second, data on diarrhoea-related hospital admissions and deaths were not available at this time due to delays when collecting information at national level. These data require further study considering the well-known impact that rotavirus vaccination produces on severe diarrhoea cases [Reference Sindhu, Babji and Ganesan7, Reference Velázquez8, Reference Desai29, Reference Richardson30]. Third, due to the procedures of data collection, we were not able to further disaggregate the 1–4 years sub-group. We notice that this aspect would be important for future studies to consider because of the high impact of rotavirus on children under 2 years of age, which could affect observations on children 3 and 4 years of age. Also, the absence of a specific surveillance system developed to monitor vaccination impact hinders collection of suitable and timely information for policy makers. This fact highlights the importance of building evidence with tools from different sources. Nonetheless, concordance between high vaccine coverage rates, the significant decrease of all-cause acute diarrhoea cases in the autumn/winter season and the sharp decline in rotavirus laboratory-confirmed cases, especially considering that each parameter is assessed with different input data, demonstrate the strong validity of our findings. Accordingly, these tools represent a suitable strategy for constant monitoring of the diarrhoeal disease burden in the post-vaccination era.
Rotavirus massive vaccination is one of the few immunisation strategies whose impact can be rapidly observed in the early years. In Argentina, its introduction into the national scheme may be considered a successful intervention due to the significant decrease in all-cause acute diarrhoea cases and rotavirus laboratory-confirmed cases in children under 5 years. Also, the rotavirus seasonal peak has blunted, and G2P[4] was the most predominant circulating genotype. Consequently, all these results lead to the supposition that the entire rotavirus circulation dynamic may be changing in our country.
Acknowledgements
Here are listed the members of Argentinean Rotavirus Surveillance Network: M. L. Benvenutti (Htal. Penna, Buenos Aires); M. F. Bulgheroni (Htal. Heller, Neuquén); G. Cabral (Htal. Posadas, Buenos Aires); F. Canna (Laboratorio Central, Córdoba); N. Cech (Htal. 4 de Junio, Chaco); S. Correa (Htal. San Luis, San Luis); P. Cortes (Htal. del Niño Jesús, Córdoba); V. Eibar (Htal. Notti, Mendoza); L. Fierro (Htal. Rawson, San Juan); S. Flores (Htal. Eva Perón, Tucumán); E. Gentini (CEMAR, Santa Fe); S. Larini (Htal. Vilela, Santa Fe); L. López (Htal. Durand, CABA); E. Lozano (Htal Quintana, Jujuy); N. Lucero (Htal. Schestakow, Mendoza); A. Millán (Htal. Alassia, Santa Fe); J. Palau (Htal. Sor María Ludovica, Buenos Aires); M. Roncallo (Htal. Cipolletti, Río Negro); L. Sánchez (CEDITET, La Rioja); I. Silveyra (Htal. Centeno, La Pampa); G. Sucin (Htal. Castelán, Chaco); A. Zurschmitten (Htal. Junín de los Andes, Neuquén).
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.








